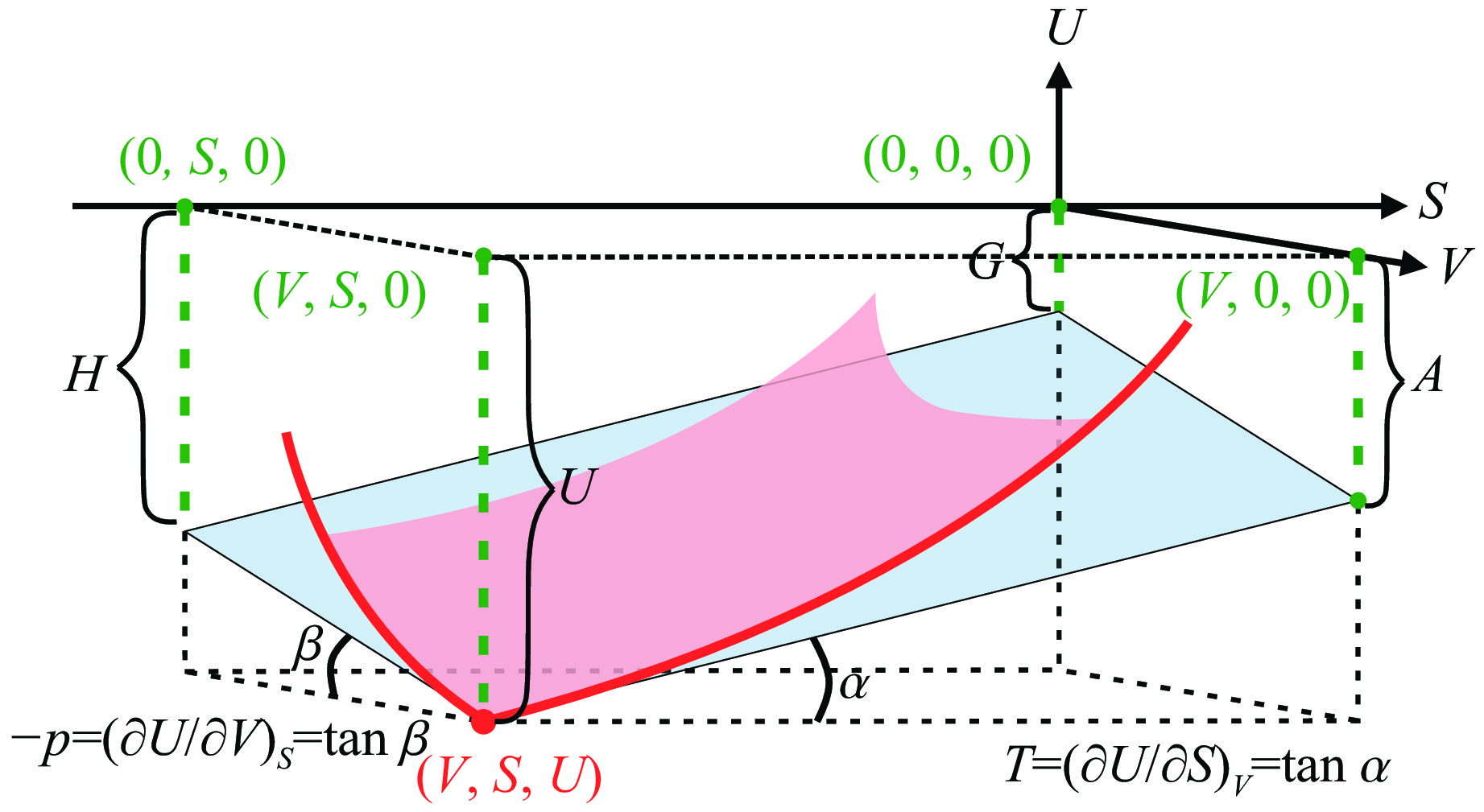
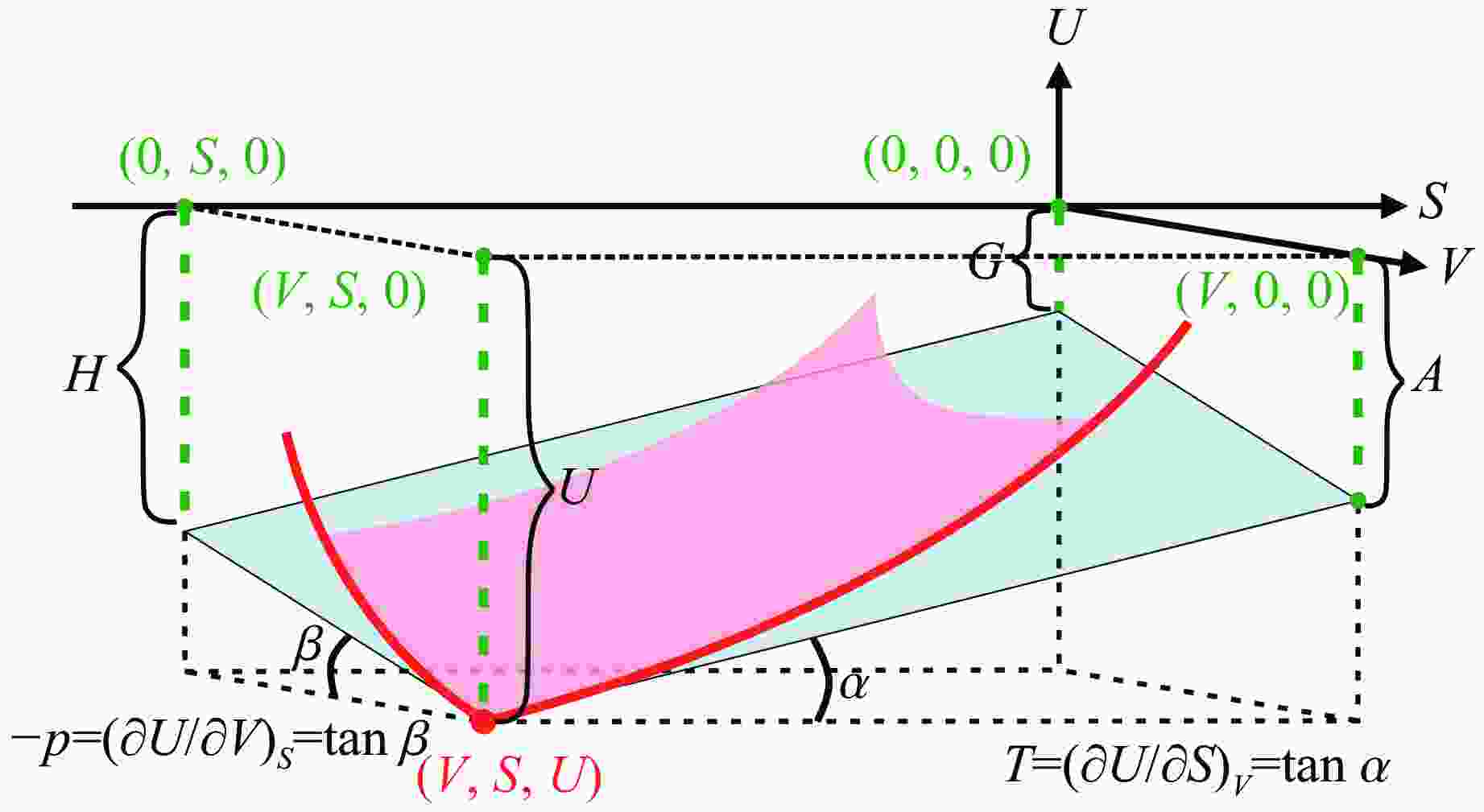
A Gibbs Thermodynamic Surface Approach to Modeling the Melting of Forsterite
-
摘要: 熔融过程在地球与其他行星的演化中发挥着重要作用。由于行星内部普遍处于高压环境,研究物质在高压条件下的熔融行为对于理解其组成及内部动力学过程至关重要。为此,在吉布斯热力学曲面理论及前人工作的基础上,结合第一性原理分子动力学模拟,通过构建几何模型,获得了镁橄榄石在0~16 GPa压力范围内的熔融数据。该方法能够在有限的计算资源下高效且准确地获取特定压力范围内任意点的熔融数据,包括固相和熔体相各自的吉布斯自由能、亥姆霍兹自由能、焓、内能、熵和体积。同时,使用该方法确定了镁橄榄石和瓦兹利石在
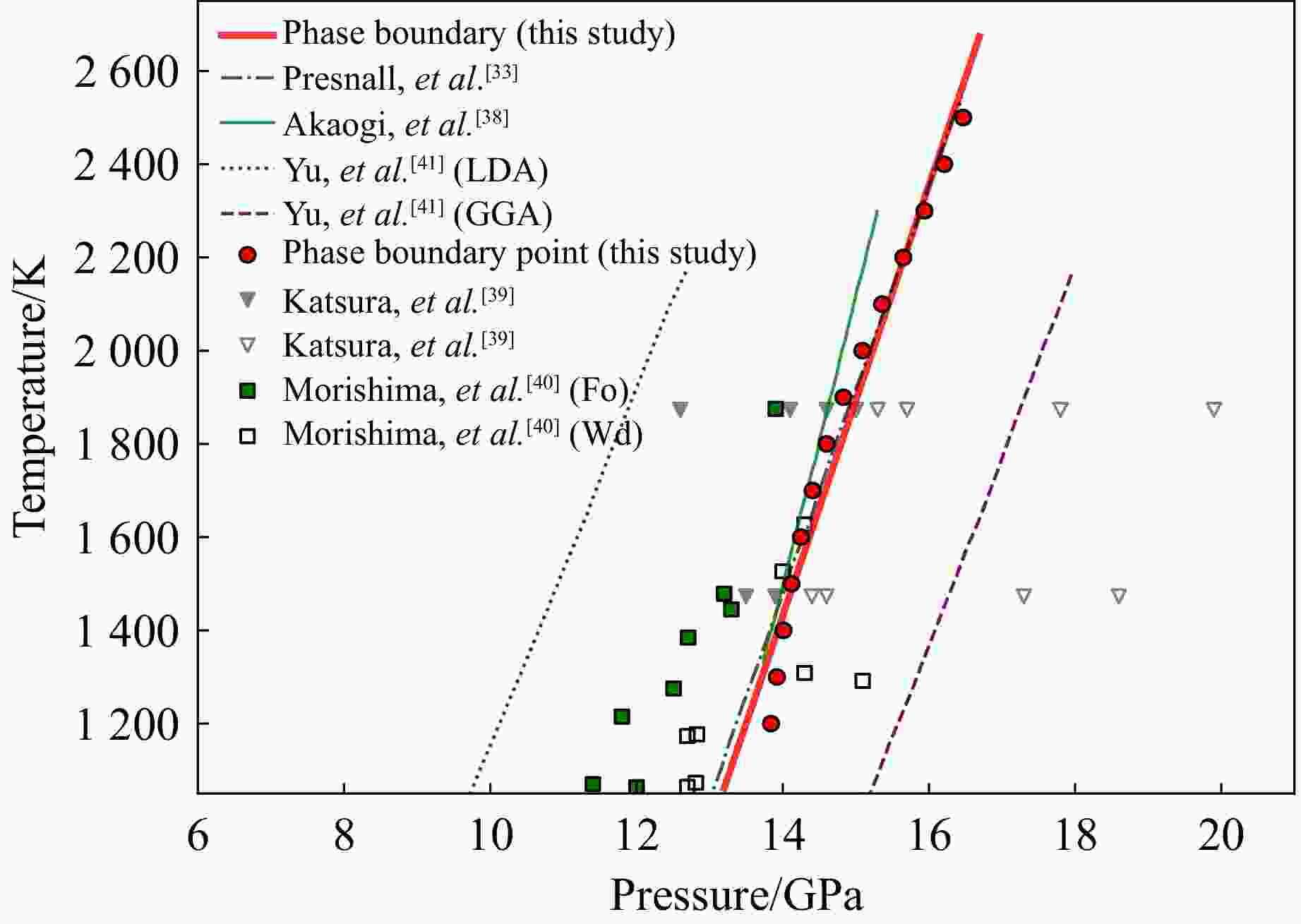
1200 ~2500 K内的相变边界。所有计算结果均与已有实验及计算数据高度一致,验证了该方法在计算高压下物质的熔化行为的可靠性和准确性。该方法突破了现有方法在有限计算资源下难以高效获取完整高压熔融数据的瓶颈。Abstract: The process of melting is widespread in nature and plays a crucial role in the evolution of magma oceans on Earth and other planetary bodies. Given that planetary interiors are generally subjected to high-pressure conditions, the study of melting behavior under high-pressure conditions is essential for understanding the composition and dynamic evolution of planetary interiors. Based on the theory of the Gibbs thermodynamic surface and previous research, this study employs ab initio molecular dynamics simulation combined with a geometric model to obtain the melting data of forsterite (Mg2SiO4) within the pressure range of 0 to 16 GPa. Under limited computational resources, this method enables the efficient and accurate acquisition of melting-related properties at any point within a given pressure range, including the Gibbs free energy, Helmholtz free energy, enthalpy, internal energy, entropy, and volume of solid and liquid phases. This approach is also used to determine the phase boundary between forsterite and wadsleyite within the temperature range of1200 to1500 K. The calculated results show high consistency with existing experimental and computational data, validating the reliability and accuracy of this method for obtaining melting data under high pressure. This approach overcomes the bottleneck of existing methods in efficiently obtaining complete high-pressure melting data with limited computational resources.-
Key words:
- melting curve /
- Gibbs thermodynamic surface /
- high pressure /
- molecular dynamics simulation /
- forsterite
-
表 1 镁橄榄石的熔融数据
Table 1. Melting data of forsterite
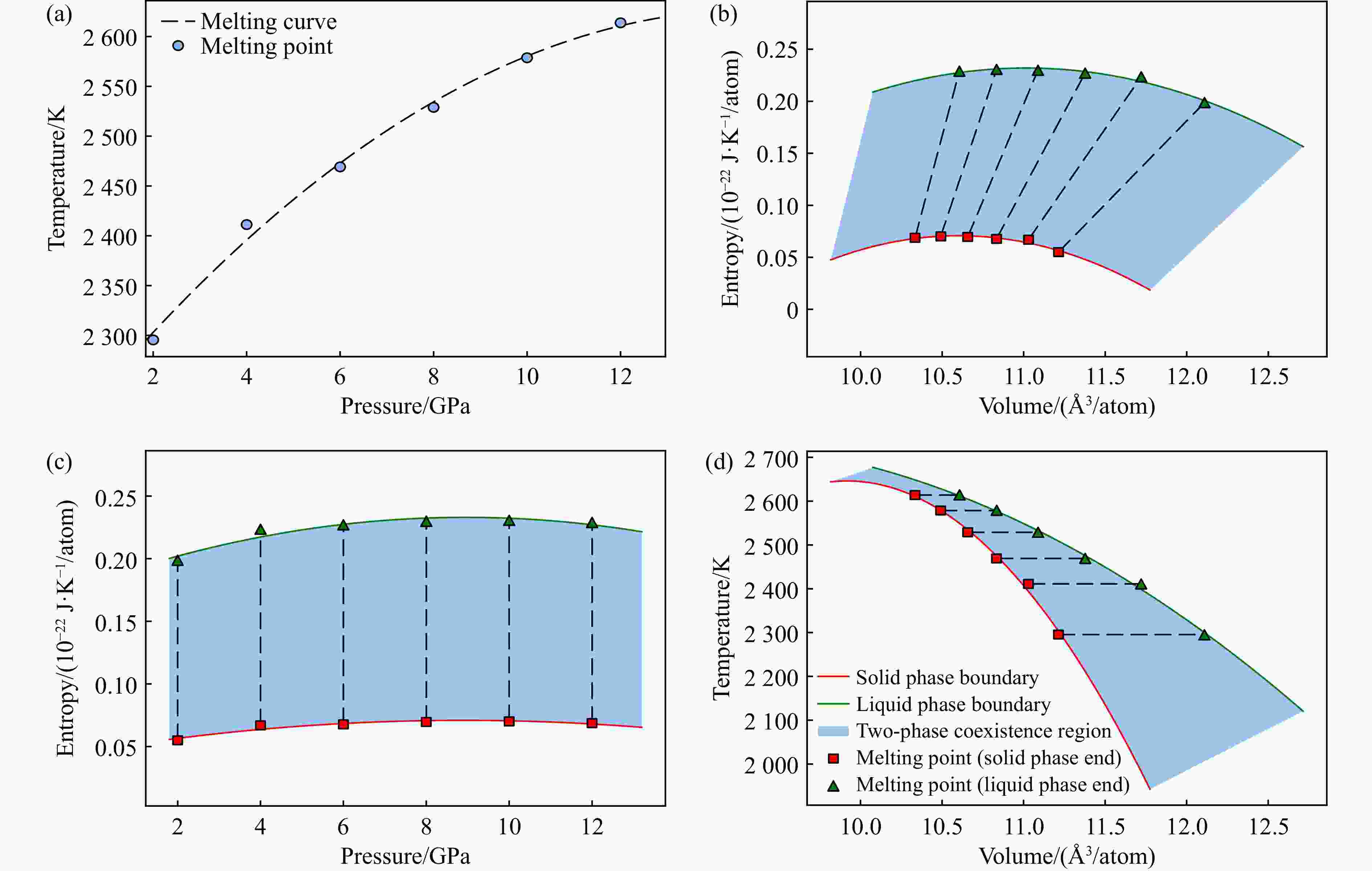
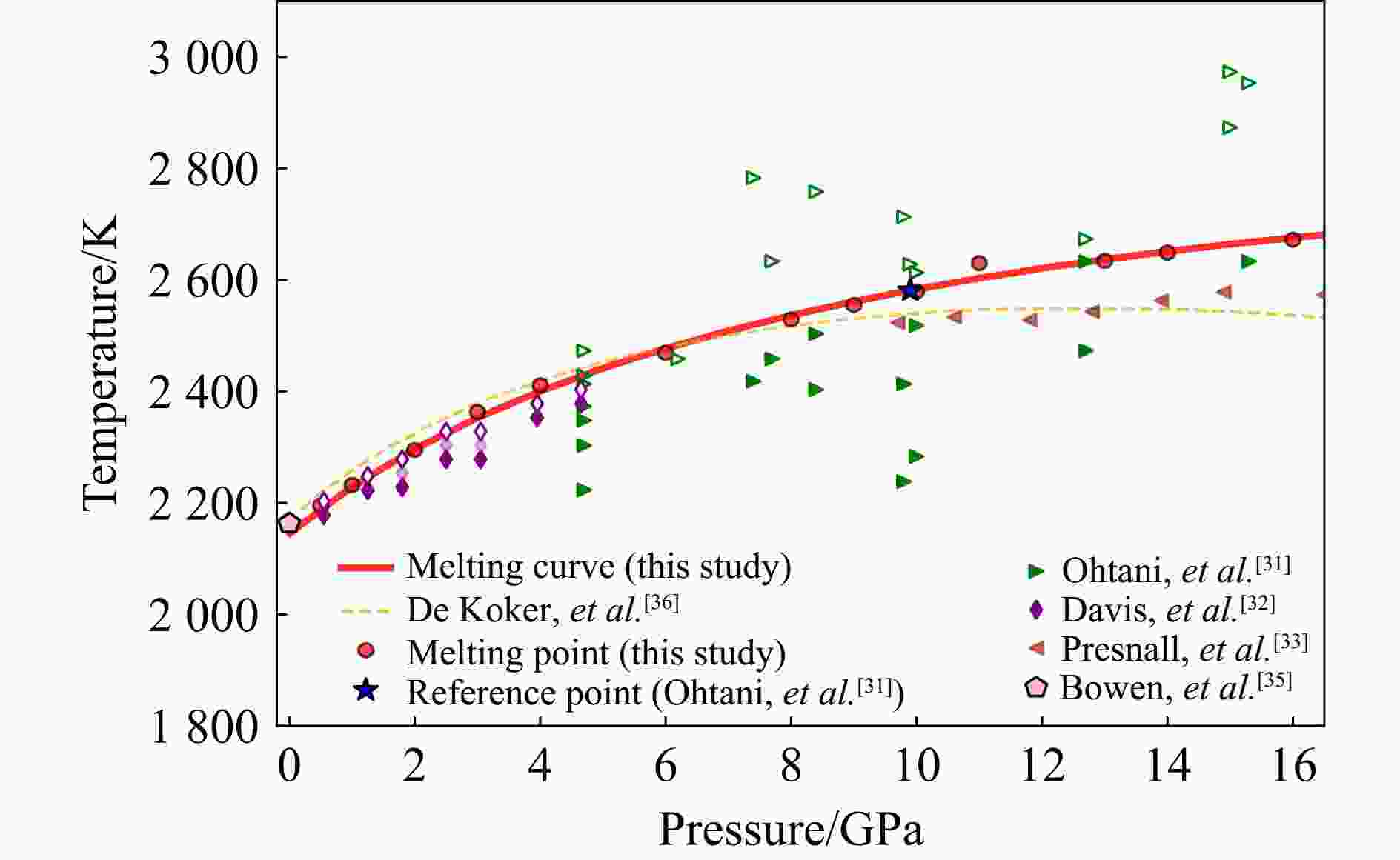
p/GPa Tm/K Phase V/(Å3/atom) G/(eV/atom) A/(eV/atom) H/(eV/atom) U/(eV/atom) S/(μeV·K−1/atom) 0.5 2195 Solid 11.357 −6.597 −6.633 −6.538 −6.574 27 0.5 2195 Liquid 12.540 −6.583 −6.622 −6.373 −6.412 102 1.0 2232 Solid 11.310 −6.563 −6.634 −6.496 −6.567 30 1.0 2232 Liquid 12.365 −6.550 −6.627 −6.318 −6.395 110 2.0 2295 Solid 11.214 −6.495 −6.635 −6.416 −6.556 34 2.0 2295 Liquid 12.109 −6.487 −6.638 −6.210 −6.361 124 3.0 2363 Solid 11.125 −6.428 −6.636 −6.335 −6.543 39 3.0 2363 Liquid 11.908 −6.425 −6.648 −6.111 −6.334 135 4.0 2411 Solid 11.125 −6.428 −6.636 −6.335 −6.543 39 4.0 2411 Liquid 11.908 −6.425 −6.648 −6.111 −6.334 135 6.0 2469 Solid 10.834 −6.226 −6.632 −6.122 −6.528 42 6.0 2469 Liquid 11.377 −6.225 −6.651 −5.876 −6.302 142 8.0 2529 Solid 10.657 −6.095 −6.627 −5.985 −6.517 43 8.0 2529 Liquid 11.088 −6.094 −6.647 −5.731 −6.285 143 9.0 2555 Solid 10.573 −6.030 −6.623 −5.918 −6.512 44 9.0 2555 Liquid 10.957 −6.029 −6.644 −5.662 −6.277 144 10.0 2579 Solid 10.492 −5.965 −6.620 −5.852 −6.507 44 10.0 2579 Liquid 10.834 −5.964 −6.641 −5.593 −6.270 144 11.0 2630 Solid 10.424 −5.902 −6.618 −5.779 −6.494 47 11.0 2630 Liquid 10.731 −5.905 −6.641 −5.517 −6.254 148 13.0 2634 Solid 10.261 −5.773 −6.606 −5.660 −6.493 43 13.0 2634 Liquid 10.503 −5.773 −6.625 −5.397 −6.249 143 14.0 2649 Solid 10.189 −5.710 −6.600 −5.598 −6.488 42 14.0 2649 Liquid 10.404 −5.710 −6.619 −5.333 −6.242 142 16.0 2672 Solid 10.051 −5.585 −6.588 −5.475 −6.479 41 16.0 2672 Liquid 10.219 −5.585 −6.605 −5.209 −6.230 140 表 2 镁橄榄石-瓦兹利石相变边界热力学数据
Table 2. Data for phase boundary between forsterite and wadsleyite
p/GPa T/K Phase V/(Å3/atom) G/(eV/atom) A/(eV/atom) H/(eV/atom) U/(eV/atom) S/(μeV·K−1/atom) 13.84 1200 Fo 9.850 −5.800 −6.651 −6.010 −6.861 −178 13.84 1200 Wd 9.259 −5.800 −6.600 −6.024 −6.824 −186 13.92 1300 Fo 9.860 −5.778 −6.635 −5.978 −6.834 −156 13.92 1300 Wd 9.277 −5.778 −6.584 −5.992 −6.798 −164 14.01 1400 Fo 9.870 −5.757 −6.621 −5.945 −6.808 −135 14.01 1400 Wd 9.296 −5.757 −6.570 −5.959 −6.772 −144 14.12 1500 Fo 9.882 −5.738 −6.608 −5.911 −6.781 −116 14.12 1500 Wd 9.315 −5.738 −6.558 −5.926 −6.747 −125 14.25 1600 Fo 9.893 −5.718 −6.598 −5.875 −6.755 −98 14.25 1600 Wd 9.333 −5.718 −6.549 −5.891 −6.721 −108 14.41 1700 Fo 9.904 −5.699 −6.590 −5.838 −6.729 −82 14.41 1700 Wd 9.352 −5.699 −6.540 −5.854 −6.695 −91 14.60 1800 Fo 9.913 −5.680 −6.583 −5.799 −6.702 −66 14.60 1800 Wd 9.368 −5.680 −6.534 −5.816 −6.670 −76 14.83 1900 Fo 9.922 −5.660 −6.578 −5.757 −6.676 −51 14.83 1900 Wd 9.383 −5.660 −6.528 −5.776 −6.644 −61 15.09 2000 Fo 9.930 −5.639 −6.574 −5.714 −6.650 −38 15.09 2000 Wd 9.395 −5.639 −6.524 −5.733 −6.618 −47 15.36 2100 Fo 9.937 −5.619 −6.572 −5.670 −6.623 −24 15.36 2100 Wd 9.405 −5.619 −6.521 −5.690 −6.592 −34 15.65 2200 Fo 9.944 −5.599 −6.571 −5.626 −6.597 −12 15.65 2200 Wd 9.413 −5.599 −6.519 −5.647 −6.566 −21 15.94 2300 Fo 9.951 −5.581 −6.571 −5.582 −6.571 0 15.94 2300 Wd 9.419 −5.581 −6.518 −5.603 −6.540 −10 16.21 2400 Fo 9.960 −5.564 −6.572 −5.538 −6.546 11 16.21 2400 Wd 9.425 −5.564 −6.518 −5.560 −6.513 2 16.47 2500 Fo 9.970 −5.550 −6.575 −5.496 −6.520 22 16.47 2500 Wd 9.429 −5.550 −6.519 −5.518 −6.487 13 -
[1] MAO H K, CHEN X J, DING Y, et al. Solids, liquids, and gases under high pressure [J]. Reviews of Modern Physics, 2018, 90(1): 015007. doi: 10.1103/RevModPhys.90.015007 [2] XU M L, LI Y W, MA Y M. Materials by design at high pressures [J]. Chemical Science, 2022, 13(2): 329–344. doi: 10.1039/D1SC04239D [3] SAKAIRI T, OHTANI E, KAMADA S, et al. Melting relations in the Fe-S-Si system at high pressure and temperature: implications for the planetary core [J]. Progress in Earth and Planetary Science, 2017, 4(1): 10. doi: 10.1186/s40645-017-0125-x [4] HU J P, SHARP T G. Formation, preservation and extinction of high-pressure minerals in meteorites: temperature effects in shock metamorphism and shock classification [J]. Progress in Earth and Planetary Science, 2022, 9(1): 6. doi: 10.1186/s40645-021-00463-2 [5] 贺芝宇, 黄秀光, 舒桦, 等. 冰巨行星内部深处物理与化学过程研究进展 [J]. 高压物理学报, 2023, 37(5): 050105. doi: 10.11858/gywlxb.20230721HE Z Y, HUANG X G, SHU H, et al. Progress on physical and chemical processes deep inside ice giants [J]. Chinese Journal of High Pressure Physics, 2023, 37(5): 050105. doi: 10.11858/gywlxb.20230721 [6] YIN K, BELONOSHKO A B, LI Y H, et al. Davemaoite as the mantle mineral with the highest melting temperature [J]. Science Advances, 2023, 9(49): eadj2660. doi: 10.1126/sciadv.adj2660 [7] KATSURA T. Phase relations of bridgmanite, the most abundant mineral in the Earth’s lower mantle [J]. Communications Chemistry, 2025, 8(1): 28. doi: 10.1038/s42004-024-01389-8 [8] LI J, WU Q, LI J B, et al. Shock melting curve of iron: a consensus on the temperature at the Earth’s inner core boundary [J]. Geophysical Research Letters, 2020, 47(15): e2020GL087758. doi: 10.1029/2020GL087758 [9] 王宝云, 肖万生, 宋茂双. δ-(Al, Fe)OOH的高压相变 [J]. 高压物理学报, 2021, 35(6): 061201. doi: 10.11858/gywlxb.20210765WANG B Y, XIAO W S, SONG M S. Pressure-induced phase transitions in δ-(Al, Fe)OOH [J]. Chinese Journal of High Pressure Physics, 2021, 35(6): 061201. doi: 10.11858/gywlxb.20210765 [10] 姜昌国, 谭大勇, 谢亚飞, 等. 高压剪切作用下三水铝石的结构稳定性 [J]. 高压物理学报, 2022, 36(1): 011202. doi: 10.11858/gywlxb.20210766JIANG C G, TAN D Y, XIE Y F, et al. Investigation on structural stability of γ-Al(OH)3 under high pressure and shear stress [J]. Chinese Journal of High Pressure Physics, 2022, 36(1): 011202. doi: 10.11858/gywlxb.20210766 [11] 陈炜珊, 谭毅, 谭大勇, 等. NaPO3高压结构行为的第一性原理理论研究 [J]. 高压物理学报, 2024, 38(5): 050106. doi: 10.11858/gywlxb.20240755CHEN W S, TAN Y, TAN D Y, et al. First-principles theoretical study on the structure behaviors of NaPO3 under compression [J]. Chinese Journal of High Pressure Physics, 2024, 38(5): 050106. doi: 10.11858/gywlxb.20240755 [12] 何宇, 孙士川, 李和平. 地球内核超离子态铁合金及其效应 [J]. 高压物理学报, 2024, 38(3): 030202. doi: 10.11858/gywlxb.20240707HE Y, SUN S C, LI H P. Superionic iron alloys in Earth’s inner core and their effects [J]. Chinese Journal of High Pressure Physics, 2024, 38(3): 030202. doi: 10.11858/gywlxb.20240707 [13] 吴忠庆, 王文忠. 矿物高温高压下弹性的第一性原理计算研究进展 [J]. 中国科学: 地球科学, 2016, 46(5): 582–617.WU Z Q, WANG W Z. First-principles calculations of elasticity of minerals at high temperature and pressure [J]. Science China Earth Sciences, 2016, 59(6): 1107–1137. [14] 甘波, 李俊, 蒋刚, 等. Fe高压熔化线的实验研究进展 [J]. 高压物理学报, 2021, 35(6): 060101. doi: 10.11858/gywlxb.20210859GAN B, LI J, JIANG G, et al. A review of the experimental determination of the melting curve of iron at ultrahigh pressures [J]. Chinese Journal of High Pressure Physics, 2021, 35(6): 060101. doi: 10.11858/gywlxb.20210859 [15] LUO S N, STRACHAN A, SWIFT D C. Nonequilibrium melting and crystallization of a model Lennard-Jones system [J]. The Journal of Chemical Physics, 2004, 120(24): 11640–11649. doi: 10.1063/1.1755655 [16] BELONOSHKO A B. Molecular dynamics of MgSiO3 perovskite at high pressures: equation of state, structure, and melting transition [J]. Geochimica et Cosmochimica Acta, 1994, 58(19): 4039–4047. doi: 10.1016/0016-7037(94)90265-8 [17] BELONOSHKO A B, SKORODUMOVA N V, ROSENGREN A, et al. Melting and critical superheating [J]. Physical Review B, 2006, 73(1): 012201. doi: 10.1103/PhysRevB.73.012201 [18] SIMON F, GLATZEL G. Bemerkungen zur schmelzdruckkurve [J]. Zeitschrift für Anorganische und Allgemeine Chemie, 1929, 178(1): 309–316. doi: 10.1002/zaac.19291780123 [19] KECHIN V V. Melting curve equations at high pressure [J]. Physical Review B, 2001, 65(5): 052102. doi: 10.1103/PhysRevB.65.052102 [20] GIBBS J W. Graphical methods in the thermodynamics of fluids [J]. Transactions of the Connecticut Academy of Arts and Sciences, 1873, 2: 309–342. [21] GIBBS J W. A method of geometrical representation of the thermodynamic properties of substances by means of surfaces [J]. Transactions of the Connecticut Academy of Arts and Sciences, 1873, 2: 382–404. [22] GIBBS J W. On the equilibrium of heterogeneous substances [J]. Transactions of the Connecticut Academy of Arts and Sciences, 1876, 3: 108–248. [23] YIN K, LU X C, ZHOU H Q, et al. Thermodynamic stability limit of the crystalline state from the Gibbs perspective [J]. Physical Review B, 2018, 98(14): 144113. doi: 10.1103/PhysRevB.98.144113 [24] KATSURA T, YAMADA H, NISHIKAWA O, et al. Olivine-wadsleyite transition in the system (Mg, Fe)2SiO4 [J]. Journal of Geophysical Research: Solid Earth, 2004, 109(B2): B02209. doi: 10.1029/2003JB002438 [25] 刘曦, 代立东, 邓力维, 等. 近十年我国在地球内部物质高压物性实验研究方面的主要进展 [J]. 高压物理学报, 2017, 31(6): 657–681. doi: 10.11858/gywlxb.2017.06.001LIU X, DAI L D, DENG L W, et al. Recent progresses in some fields of high-pressure physics relevant to Earth sciences achieved by Chinese scientists [J]. Chinese Journal of High Pressure Physics, 2017, 31(6): 657–681. doi: 10.11858/gywlxb.2017.06.001 [26] BOSTROEM D. Single-crystal X-ray diffraction studies of synthetic Ni-Mg olivine solid solutions [J]. American Mineralogist, 1987, 72(9/10): 965–972. [27] KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set [J]. Physical Review B, 1996, 54(16): 11169–11186. doi: 10.1103/PhysRevB.54.11169 [28] PERDEW J P, RUZSINSZKY A, CSONKA G I, et al. Restoring the density-gradient expansion for exchange in solids and surfaces [J]. Physical Review Letters, 2008, 100(13): 136406. doi: 10.1103/PhysRevLett.100.136406 [29] NOSÉ S. A unified formulation of the constant temperature molecular dynamics methods [J]. The Journal of Chemical Physics, 1984, 81(1): 511–519. doi: 10.1063/1.447334 [30] HOOVER W G. Canonical dynamics: equilibrium phase-space distributions [J]. Physical Review A, 1985, 31(3): 1695–1697. doi: 10.1103/PhysRevA.31.1695 [31] OHTANI E, KUMAZAWA M. Melting of forsterite Mg2SiO4 up to 15 GPa [J]. Physics of the Earth and Planetary Interiors, 1981, 27(1): 32–38. doi: 10.1016/0031-9201(81)90084-4 [32] DAVIS B T C, ENGLAND J L. The melting of forsterite up to 50 kilobars [J]. Journal of Geophysical Research, 1964, 69(6): 1113–1116. doi: 10.1029/JZ069i006p01113 [33] PRESNALL D C, WALTER M J. Melting of forsterite, Mg2SiO4, from 9.7 to 16.5 GPa [J]. Journal of Geophysical Research: Solid Earth, 1993, 98(B11): 19777–19783. doi: 10.1029/93JB01007 [34] BOYD F R, ENGLAND J L. The quartz-coesite transition [J]. Journal of Geophysical Research, 1960, 65(2): 749–756. doi: 10.1029/JZ065i002p00749 [35] BOWEN N L, ANDERSEN O. The binary system MgO-SiO2 [J]. American Journal of Science, 1914, s4-37(222): 487–500. [36] DE KOKER N P, STIXRUDE L, KARKI B B. Thermodynamics, structure, dynamics, and freezing of Mg2SiO4 liquid at high pressure [J]. Geochimica et Cosmochimica Acta, 2008, 72(5): 1427–1441. doi: 10.1016/j.gca.2007.12.019 [37] HORIUCHI H, SAWAMOTO H. β-Mg2SiO4: single-crystal X-ray diffraction study [J]. American Mineralogist, 1981, 66(5/6): 568–575. [38] AKAOGI M, ITO E, NAVROTSKY A. Olivine-modified spinel-spinel transitions in the system Mg2SiO4-Fe2SiO4: calorimetric measurements, thermochemical calculation, and geophysical application [J]. Journal of Geophysical Research: Solid Earth, 1989, 94(B11): 15671–15685. doi: 10.1029/JB094iB11p15671 [39] KATSURA T, ITO E. The system Mg2SiO4-Fe2SiO4 at high pressures and temperatures: precise determination of stabilities of olivine, modified spinel, and spinel [J]. Journal of Geophysical Research: Solid Earth, 1989, 94(B11): 15663–15670. doi: 10.1029/JB094iB11p15663 [40] MORISHIMA H, KATO T, SUTO M, et al. The phase boundary between α- and β-Mg2SiO4 determined by in situ X-ray observation [J]. Science, 1994, 265(5176): 1202–1203. doi: 10.1126/science.265.5176.1202 [41] YU Y G, WU Z Q, WENTZCOVITCH R M. α-β-γ transformations in Mg2SiO4 in Earth’s transition zone [J]. Earth and Planetary Science Letters, 2008, 273(1/2): 115–122. doi: 10.1016/j.jpgl.2008.06.023 [42] GASPARIK T. Phase relations in the transition zone [J]. Journal of Geophysical Research: Solid Earth, 1990, 95(B10): 15751–15769. doi: 10.1029/JB095iB10p15751 [43] PERDEW J P, ZUNGER A. Self-interaction correction to density-functional approximations for many-electron systems [J]. Physical Review B, 1981, 23(10): 5048–5079. doi: 10.1103/PhysRevB.23.5048 [44] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865 -






 下载:
下载: