A First-Principles Study of Indium Migration in ZnS Minerals
doi: 10.11858/gywlxb.20251096
-
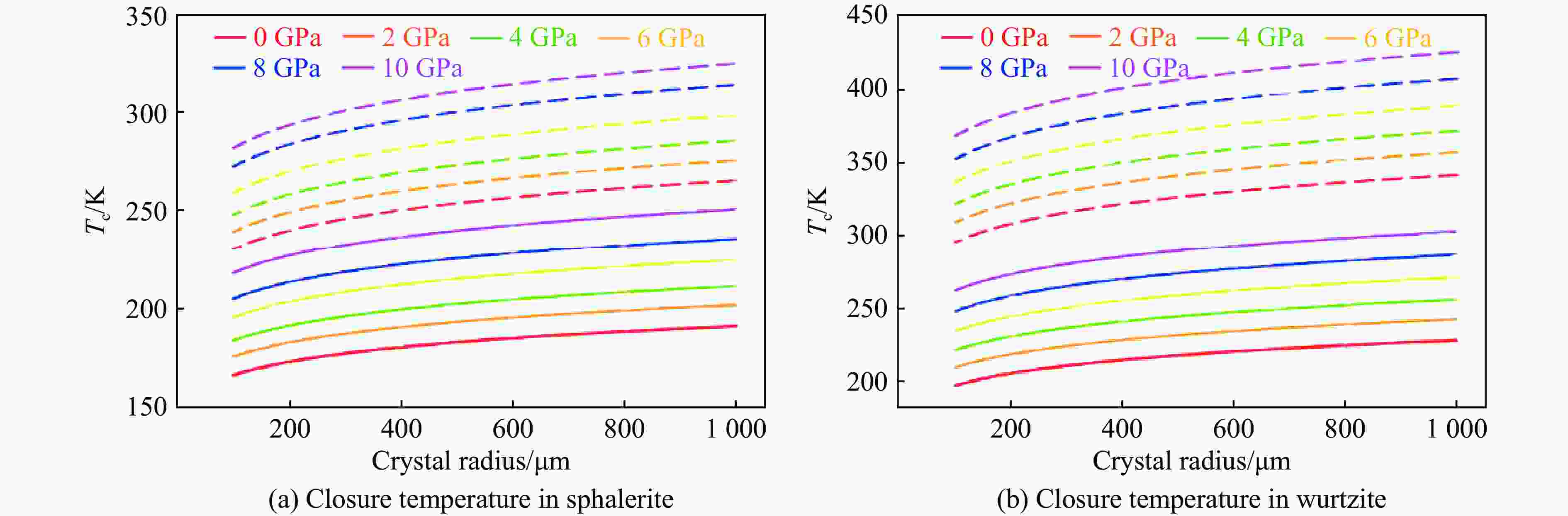
摘要: 揭示铟在ZnS矿物中的扩散机制有助于阐明铟在典型赋铟矿物中迁移、富集或贫化的动力学过程,为高品质铟矿床勘查提供理论依据。为此,对闪锌矿和纤锌矿进行了研究,确定了铟的稳定赋存位点及扩散路径,基于第一性原理计算,结合CI-NEB方法,系统计算了铟在2类ZnS矿物中的输运性质。结果表明,晶体结构的各向异性能够显著调控铟的扩散特性,其中,纤锌矿表现出更强的扩散方向依赖性以及高于闪锌矿的铟滞留能力。在0~10 GPa压力范围内,铟在纤锌矿中的扩散各向异性程度(较闪锌矿高2~3个数量级)更显著,且扩散速率始终低于闪锌矿。此外,封闭温度的计算结果表明,闪锌矿[111]方向(较[110]方向高约65 K)和纤锌矿[001]方向(较[100]方向高约100 K)具有更高的封闭阈值,且纤锌矿整体封闭温度高于闪锌矿。计算结果显示,相较于闪锌矿,纤锌矿滞留铟的能力更强,其可能是一种潜在的铟的关键寄主矿物。研究结果对于理解铟的地球化学循环以及矿产勘查与成矿研究具有一定的指导意义。Abstract: Understanding the diffusion mechanisms of indium (In) in ZnS minerals can clarify the kinetic processes governing its migration, enrichment, or depletion in these typical In-host minerals, thereby establishing a theoretical foundation for the exploration of high-grade In deposits. This study investigates sphalerite and wurtzite to identify stable In incorporation sites and diffusion pathways, and systematically calculates In transport properties in two types of ZnS minerals using first-principles calculations combined with the climbing image-nudged elastic band (CI-NEB) method. The results demonstrate that structural anisotropy significantly governs In diffusion characteristics, with wurtzite exhibiting stronger direction-dependent diffusion behavior and superior In retention capacity compared to sphalerite. Across the 0−10 GPa pressure range, In diffusion in wurtzite shows markedly higher anisotropy (2−3 orders of magnitude greater than in sphalerite) and consistently lower diffusion rates. Furthermore, closure temperature calculations reveal spatial heterogeneity, with the [111] direction in sphalerite (about 65 K higher than [110] direction) and the [001] direction in wurtzite (about 100 K higher than [100] direction) displaying elevated closure thresholds. Overall, wurtzite achieves higher closure temperatures than sphalerite. These computational findings indicate that wurtzite exhibits stronger In retention capabilities than sphalerite, suggesting its potential as a critical host mineral for In. These insights provide valuable implications for understanding In geochemical cycling and offer some guidance for mineral exploration and ore genesis studies.
-
Key words:
- indium /
- diffusion /
- sphalerite /
- wurtzite /
- anisotropy /
- closure temperature
-
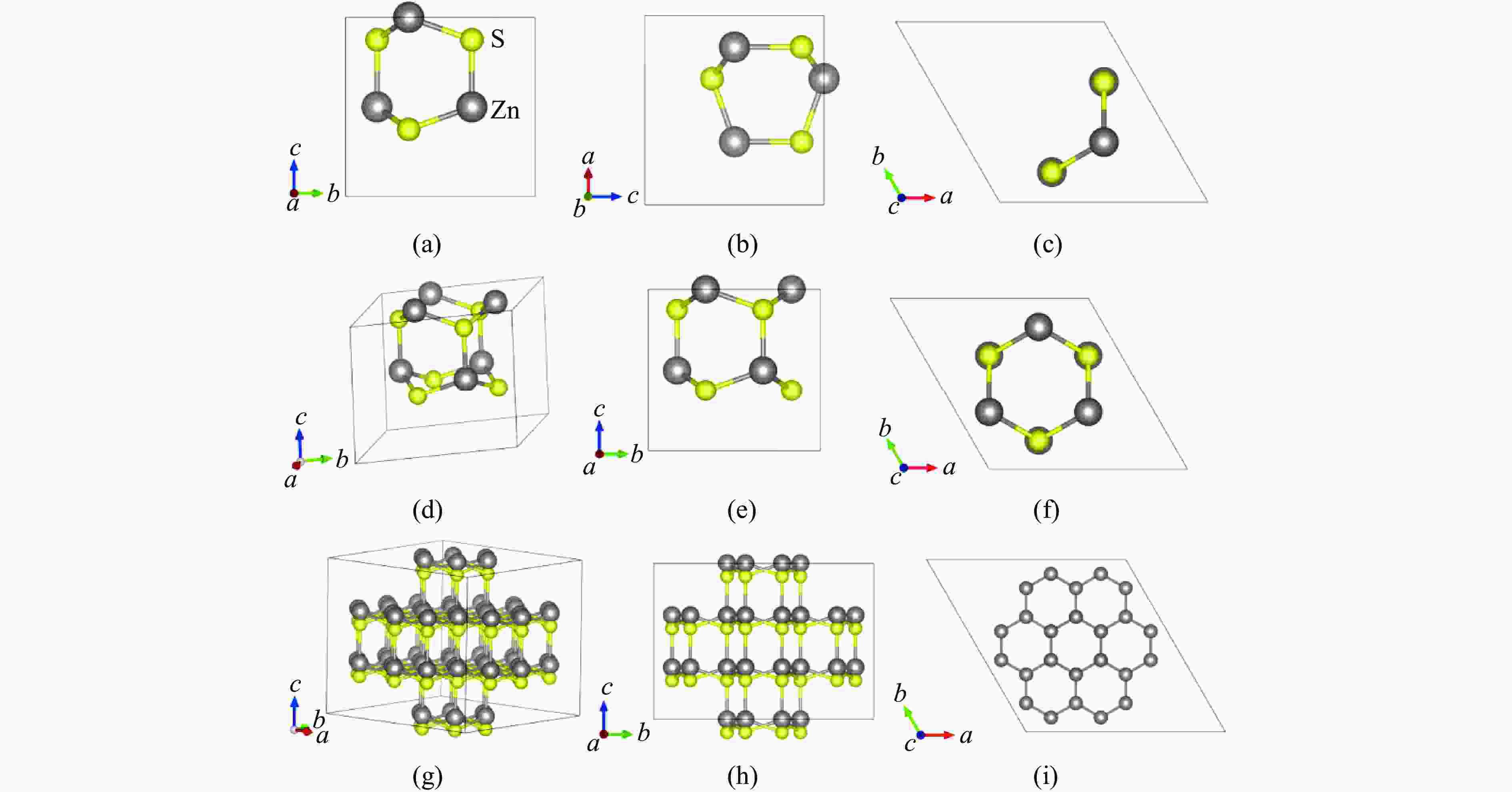
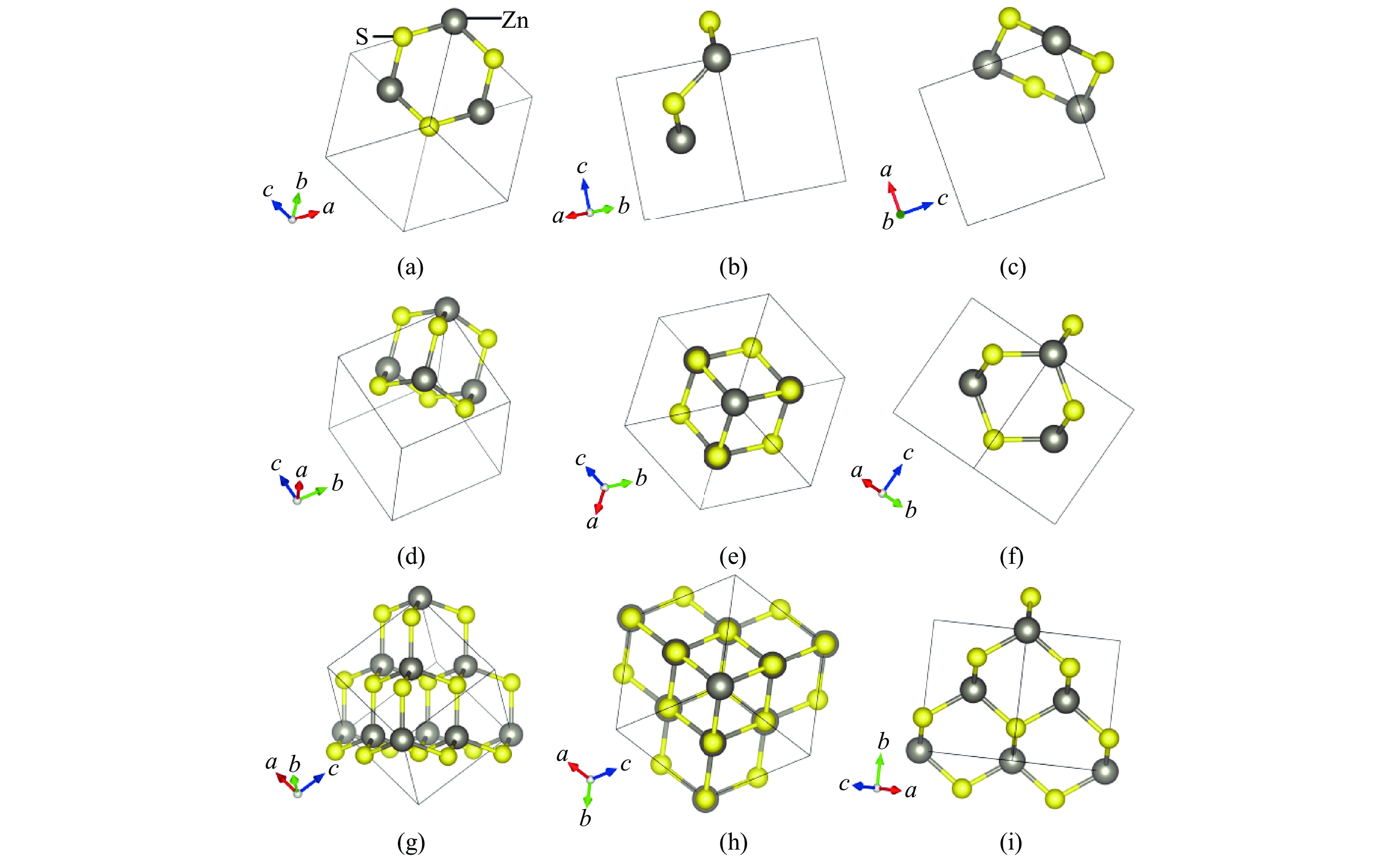
Figure 1. Structure of sphalerite: (a)−(c) the [111], [110], and [010] perspectives of the “chair-like” structure hexagonal rings; (d) the “small cage” structure; (e)−(f) the [111] and [110] perspectives of the “small cage” structure; (g) each “small cage” and its four adjacent “small cages”; (h)−(i) the [111] and [101] perspectives of each “small cage” and its four adjacent “small cages”
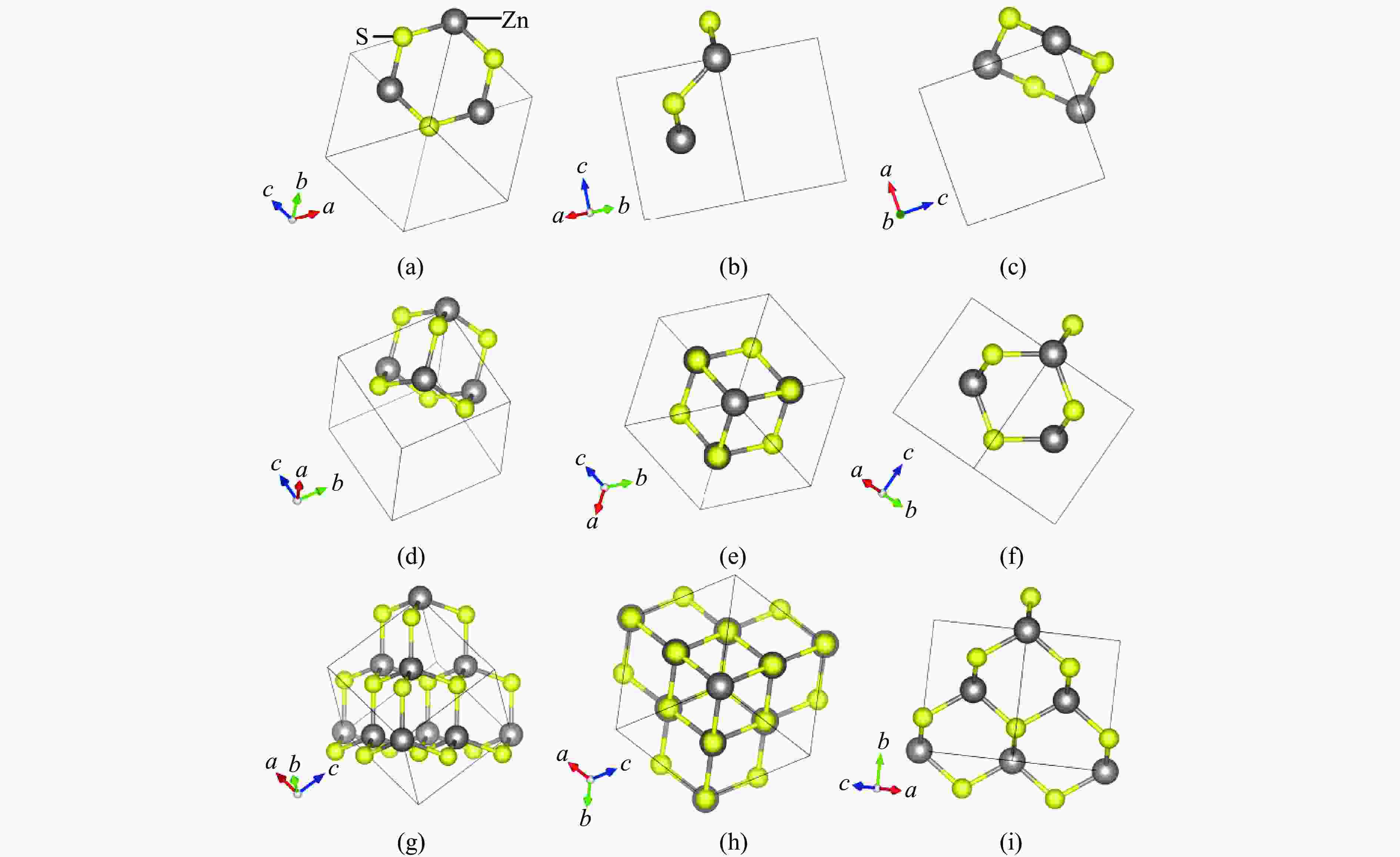
Figure 3. Structure of wurtzite: (a)−(c) the [100], [010], and [001] perspectives of the “boat-like” structure hexagonal rings; (d) the “small cage” structure; (e)−(f) the [100] and [001] perspectives of the “small cage” structure; (g) each “small cage” and its eight adjacent “small cages”; (h)−(i) the [100] and [001] perspectives of each “small cage” and its eight adjacent “small cages”
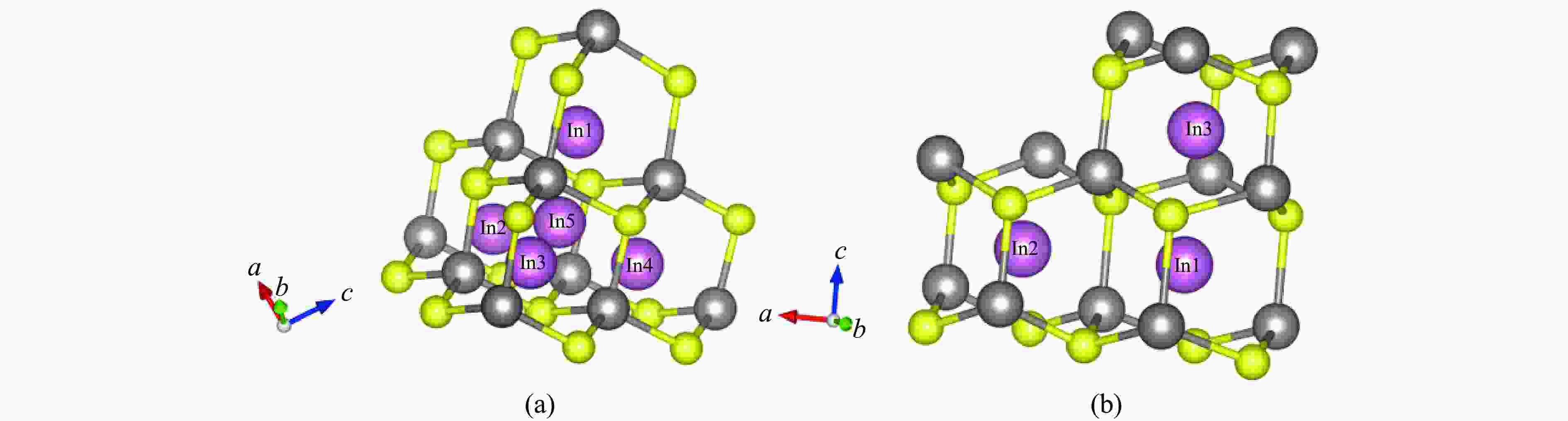
Table 1. Positions of In3+ ions in these two types of ZnS before structural optimization
ZnS type Atom Position Sphalerite
(cubic ZnS)In1 ( 0.87500 ,0.87500 ,0.87500 )In2 ( 0.87500 ,0.62500 ,0.62500 )In3 ( 0.62500 ,0.87500 ,0.62500 )In4 ( 0.62500 ,0.62500 ,0.87500 )In5 ( 0.75000 ,0.75000 ,0.75000 )Wurtzite
(hexagonal ZnS)In1 ( 0.33333 ,0.66666 ,0.59370 )In2 ( 0.67075 ,0.66529 ,0.59370 )In3 ( 0.33333 ,0.66666 ,0.84797 )Table 2. Stable positions of In3+ ions in these two types of ZnS after structural optimization
ZnS type Atom Position Sphalerite
(cubic ZnS)In1 ( 0.87500 ,0.87499 ,0.87499 )In2 ( 0.87500 ,0.62499 ,0.62499 )In3 ( 0.62501 ,0.87498 ,0.62501 )In4 ( 0.62499 ,0.62499 ,0.87499 )In5 ( 0.74999 ,0.75000 ,0.75001 )Wurtzite
(hexagonal ZnS)In1 ( 0.33333 ,0.66667 ,0.59147 )In2 ( 0.66748 ,0.66713 ,0.59189 )In3 ( 0.33333 ,0.66666 ,0.84303 )Table 3. Parameters of In diffusion in ZnS under high pressure up to 10 GPa
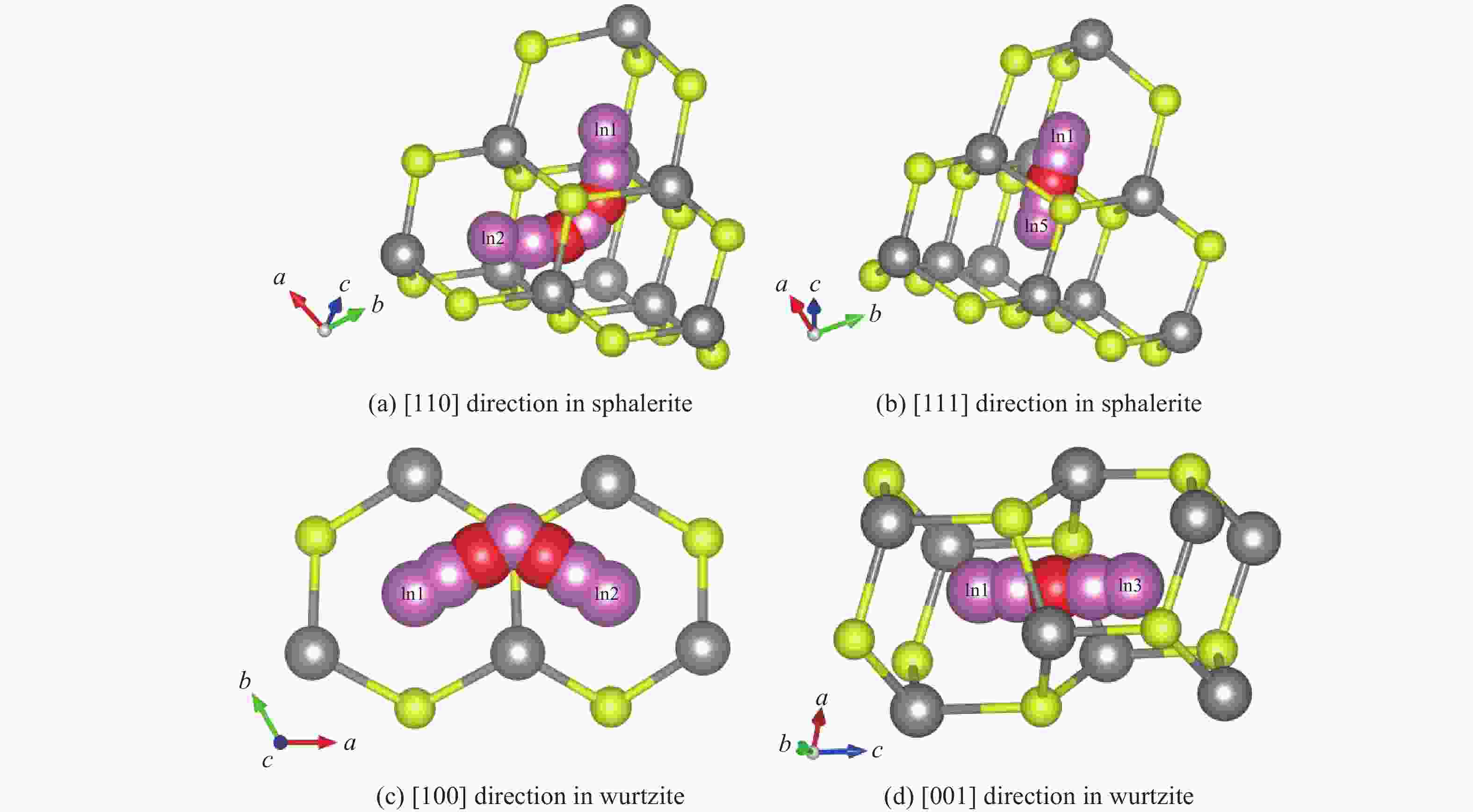
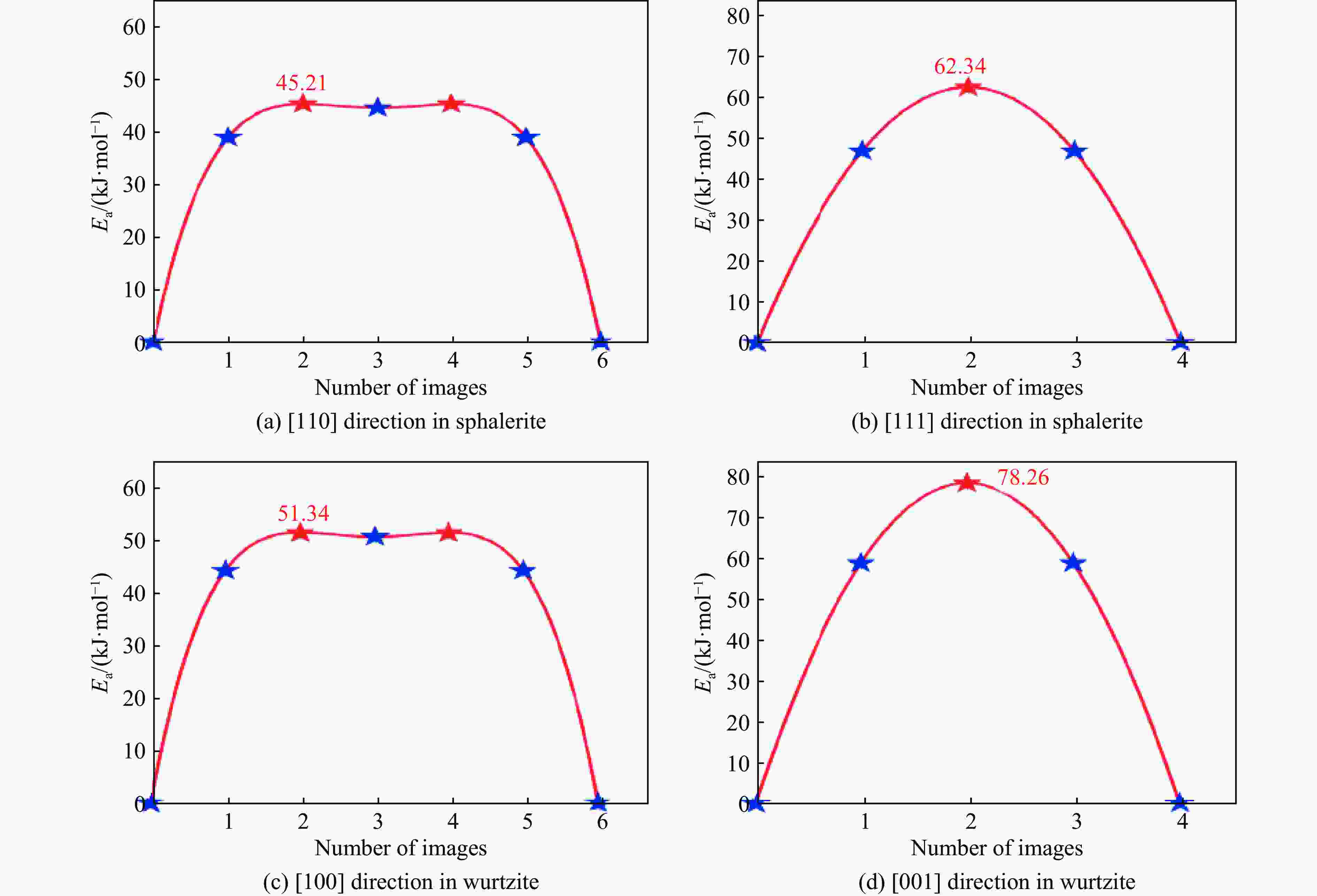
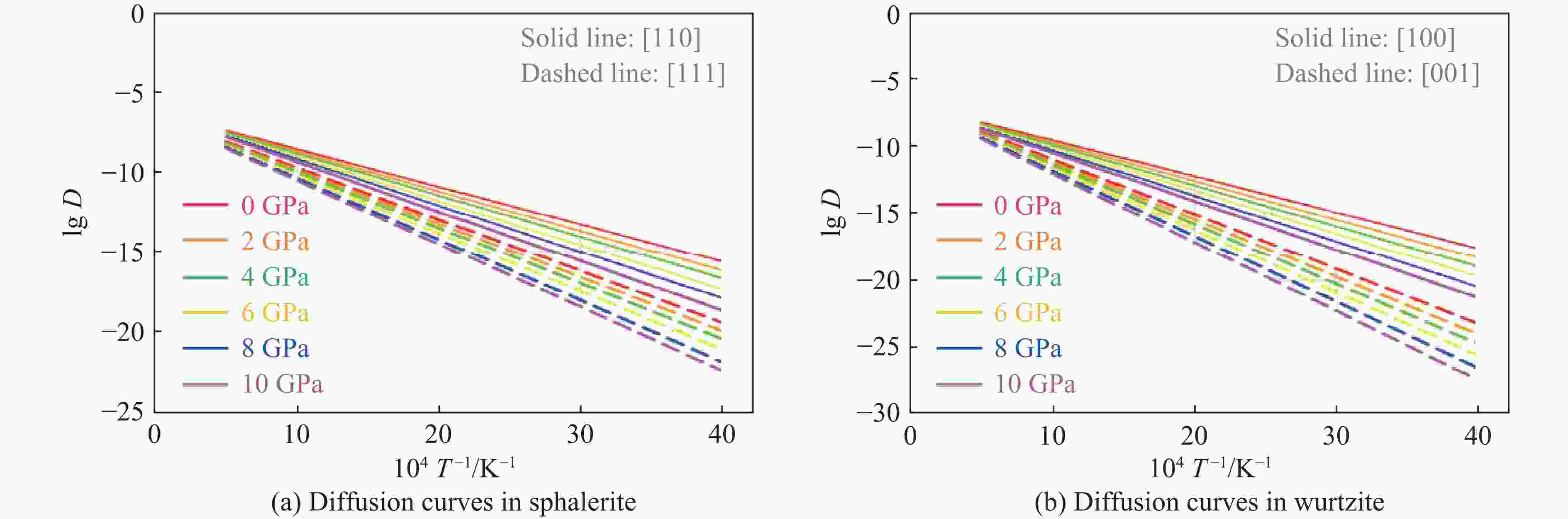
ZnS type Pressure/GPa Direction Ea/(kJ·mol−1) ν/THz l/Å D0/(m2·s−1) Sphalerite
(cubic ZnS)0 [110] 45.21 8.50 3.85 6.29×10−7 0 [111] 62.34 12.30 2.36 3.42×10−7 2 [110] 47.83 8.62 3.78 6.16×10−7 2 [111] 64.78 12.45 2.31 3.32×10−7 4 [110] 50.15 8.75 3.72 6.05×10−7 4 [111] 67.15 12.60 2.26 3.22×10−7 6 [110] 53.42 8.85 3.65 5.89×10−7 6 [111] 70.22 12.75 2.21 3.11×10−7 8 [110] 56.09 9.02 3.59 5.81×10−7 8 [111] 73.89 12.90 2.17 3.04×10−7 10 [110] 59.77 9.15 3.53 5.70×10−7 10 [111] 76.45 13.05 2.12 2.93×10−7 Wurtzite
(hexagonal ZnS)0 [100] 51.34 1.65 3.86 1.23×10−7 0 [001] 78.26 2.40 3.17 1.21×10−7 2 [100] 54.72 1.70 3.79 1.22×10−7 2 [001] 81.94 2.48 3.11 1.20×10−7 4 [100] 57.88 1.75 3.72 1.21×10−7 4 [001] 85.37 2.56 3.05 1.19×10−7 6 [100] 61.45 1.82 3.65 1.21×10−7 6 [001] 89.45 2.65 2.99 1.18×10−7 8 [100] 65.13 1.88 3.58 1.20×10−7 8 [001] 93.82 2.73 2.93 1.17×10−7 10 [100] 69.02 1.95 3.51 1.20×10−7 10 [001] 98.16 2.81 2.87 1.16×10−7 -
[1] VAUGHAN D J, CORKHILL C L. Mineralogy of sulfides [J]. Elements, 2017, 13(2): 81–87. doi: 10.2113/gselements.13.2.81 [2] GALSIN J S. Crystal structure of solids [M]//GALSIN J S. Solid State Physics: An Introduction to Theory. Waltham: Academic Press, 2019: 1–36. [3] ZHAO T P, CHEN C, HE X H, et al. A synthesis of the geology, spatial-temporal distribution and enrichment mechanism of granite-related indium deposits in China [J]. Ore Geology Reviews, 2022, 146: 104932. doi: 10.1016/j.oregeorev.2022.104932 [4] COOK N J, CIOBANU C L, PRING A, et al. Trace and minor elements in sphalerite: a LA-ICPMS study [J]. Geochimica et Cosmochimica Acta, 2009, 73(16): 4761–4791. doi: 10.1016/j.gca.2009.05.045 [5] CLOSS L G, SCHWARZ-SCHAMPERA U, HERZIG P M. Indium: geology, mineralogy, and economics [J]. Mineralium Deposita, 2003, 38(7): 913. doi: 10.1007/s00126-003-0357-0 [6] KIM H, SHIN D, IM H, et al. Distribution of indium and gallium in sphalerite from skarn and hydrothermal vein deposits in the Hwanggangri mineralized district, South Korea [J]. Journal of Geochemical Exploration, 2024, 259: 107418. doi: 10.1016/j.gexplo.2024.107418 [7] YANG X, LI Y Q, CHEN J H. DFT study of the occurrence state of In and the correlation of In and Fe in sphalerite [J]. Minerals Engineering, 2022, 183: 107596. doi: 10.1016/j.mineng.2022.107596 [8] WANG S L, LIU H X, YANG Z N. Anisotropic low-field electron diffusion coefficient and mobility in wurtzite indium nitride [J]. Physica Status Solidi B, 2014, 251(1): 168–171. doi: 10.1002/pssb.201349085 [9] DUAN H C, HUANG F. Equilibrium indium isotope fractionation in chloride-rich aqueous solutions using first-principles calculations [J]. Geochimica et Cosmochimica Acta, 2025, 393: 304–317. doi: 10.1016/j.gca.2025.01.026 [10] WANG K, BRODHOLT J, LU X C. Helium diffusion in olivine based on first principles calculations [J]. Geochimica et Cosmochimica Acta, 2015, 156: 145–153. doi: 10.1016/j.gca.2015.01.023 [11] LI S C, LIU H, YANG Y C, et al. Diffusion of helium in calcite and aragonite: a first-principles study [J]. Chinese Journal of High Pressure Physics, 2019, 33(5): 052202. doi: 10.11858/gywlxb.20180698 [12] LIU H, WANG L L, LI S C, et al. A first-principles study of helium diffusion in quartz and coesite under high pressure up to 12 GPa [J]. Geoscience Frontiers, 2021, 12(2): 1001–1009. doi: 10.1016/j.gsf.2020.09.009 [13] WANG K, LU X C, BRODHOLT J P. Diffusion of noble gases in subduction zone hydrous minerals [J]. Geochimica et Cosmochimica Acta, 2020, 291: 50–61. doi: 10.1016/j.gca.2020.07.015 [14] FIGOWY S, MOHN C E, CARACAS R. Noble gas migration in silica polymorphs at Earth’s mantle conditions [J]. Earth and Planetary Science Letters, 2024, 633: 118637. doi: 10.1016/j.jpgl.2024.118637 [15] CHEN C, ZHAO T P. Metallogenesis of indium in magmatic hydrothermal system [J]. Mineral Deposits, 2021, 40(2): 206–220. doi: 10.16111/j.0258-7106.2021.02.002 [16] MCINTYRE N S, CABRI L J, CHAUVIN W J, et al. Secondary ion mass spectrometric study of dissolved silver and indium in sulfide minerals [J]. Scanning Electron Microscopy, 1984, 3: 1139–1146. [17] JOHAN Z. Indium and germanium in the structure of sphalerite: an example of coupled substitution with copper [J]. Mineralogy and Petrology, 1988, 39(3): 211–229. doi: 10.1007/BF01163036 [18] MURAKAMI H, ISHIHARA S. Trace elements of indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: a femto-second LA-ICPMS study [J]. Ore Geology Reviews, 2013, 53: 223–243. doi: 10.1016/j.oregeorev.2013.01.010 [19] XU J, LI X F. Spatial and temporal distributions, metallogenic backgrounds and processes of indium deposits [J]. Acta Petrologica Sinica, 2018, 34(12): 3611–3626. [20] FILIMONOVA O N, TRIGUB A L, TONKACHEEV D E, et al. Substitution mechanisms in In-, Au-, and Cu-bearing sphalerites studied by X-ray absorption spectroscopy of synthetic compounds and natural minerals [J]. Mineralogical Magazine, 2019, 83(3): 435–451. doi: 10.1180/mgm.2019.10 [21] ZHOU Z B, WEN H J, QIN C J, et al. Geochemical and isotopic evidence for a magmatic-hydrothermal origin of the polymetallic vein-type Zn-Pb deposits in the northwest margin of Jiangnan Orogen, South China [J]. Ore Geology Reviews, 2017, 86: 673–691. doi: 10.1016/j.oregeorev.2017.03.022 [22] HE X H, YOU Y Y, LI W T, et al. The enrichment mechanism of indium in Fe-enriched sphalerite from the Bainiuchang Zn-Sn polymetallic deposit, SW China [J]. Ore Geology Reviews, 2024, 167: 105981. doi: 10.1016/j.oregeorev.2024.105981 [23] XIAO F, LIN W P, CHENG Q M. Ab-initio calculations and molecular dynamics simulations of In, Ag, and Cu replacing Zn in sphalerite: implications for critical metal mineralization [J]. Ore Geology Reviews, 2023, 163: 105699. doi: 10.1016/j.oregeorev.2023.105699 [24] HE Z C, XIAO F, CHENG Q M. Substitution of In and Cu for Zn in wurtzite and sphalerite with implications for ore genesis: insights from ab initio calculations and molecular dynamics simulations [J]. Journal of Asian Earth Sciences, 2025, 279: 106460. doi: 10.1016/j.jseaes.2024.106460 [25] HOHENBERG P, KOHN W. Density functional theory (DFT) [J]. Physical Review, 1964, 136(3B): B864–B871. doi: 10.1103/PhysRev.136.B864 [26] KOHN W, SHAM L J. Self-consistent equations including exchange and correlation effects [J]. Physical Review, 1965, 140(4A): A1133–A1138. doi: 10.1103/PhysRev.140.A1133 [27] KRESSE G, HAFNER J. Ab initio molecular dynamics for open-shell transition metals [J]. Physical Review B, 1993, 48(17): 13115–13118. doi: 10.1103/PhysRevB.48.13115 [28] KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set [J]. Physical Review B, 1996, 54(16): 11169–11186. doi: 10.1103/physrevb.54.11169 [29] BLÖCHL P E. Projector augmented-wave method [J]. Physical Review B, 1994, 50(24): 17953–17979. doi: 10.1103/PhysRevB.50.17953 [30] KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method [J]. Physical Review B, 1999, 59(3): 1758–1775. doi: 10.1103/PhysRevB.59.1758 [31] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865 [32] CHADI D J. Special points for Brillouin-zone integrations [J]. Physical Review B, 1977, 16(4): 1746–1747. doi: 10.1103/PhysRevB.16.1746 [33] HENKELMAN G, UBERUAGA B P, JÓNSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths [J]. The Journal of Chemical Physics, 2000, 113(22): 9901–9904. doi: 10.1063/1.1329672 [34] VINEYARD G H. Frequency factors and isotope effects in solid state rate processes [J]. Journal of Physics and Chemistry of Solids, 1957, 3(1/2): 121–127. doi: 10.1016/0022-3697(57)90059-8 [35] DODSON M H. Closure temperature in cooling geochronological and petrological systems [J]. Contributions to Mineralogy and Petrology, 1973, 40(3): 259–274. doi: 10.1007/BF00373790 [36] FARLEY K A. Helium diffusion from apatite: general behavior as illustrated by Durango fluorapatite [J]. Journal of Geophysical Research: Solid Earth, 2000, 105(B2): 2903–2914. doi: 10.1029/1999JB900348 -






 下载:
下载: