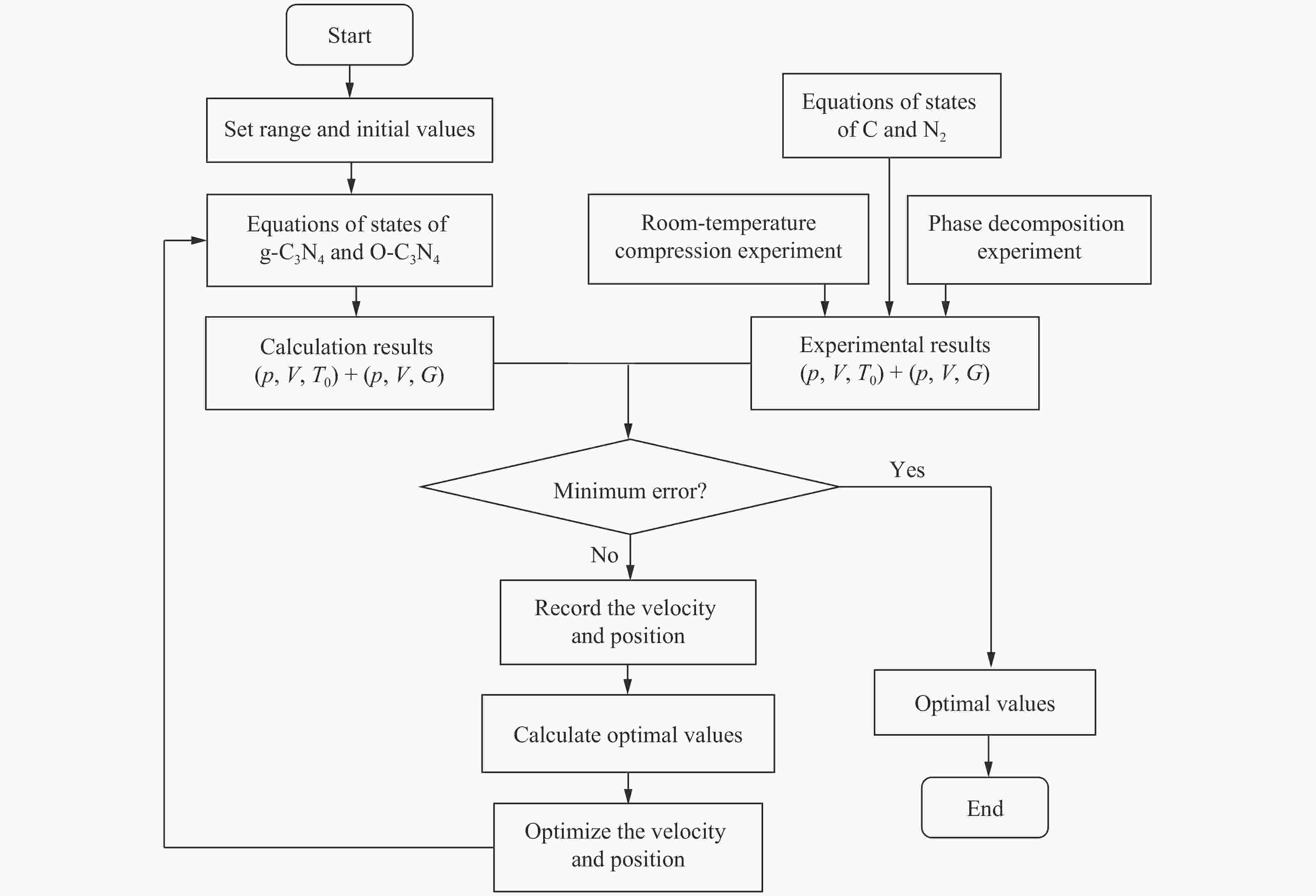
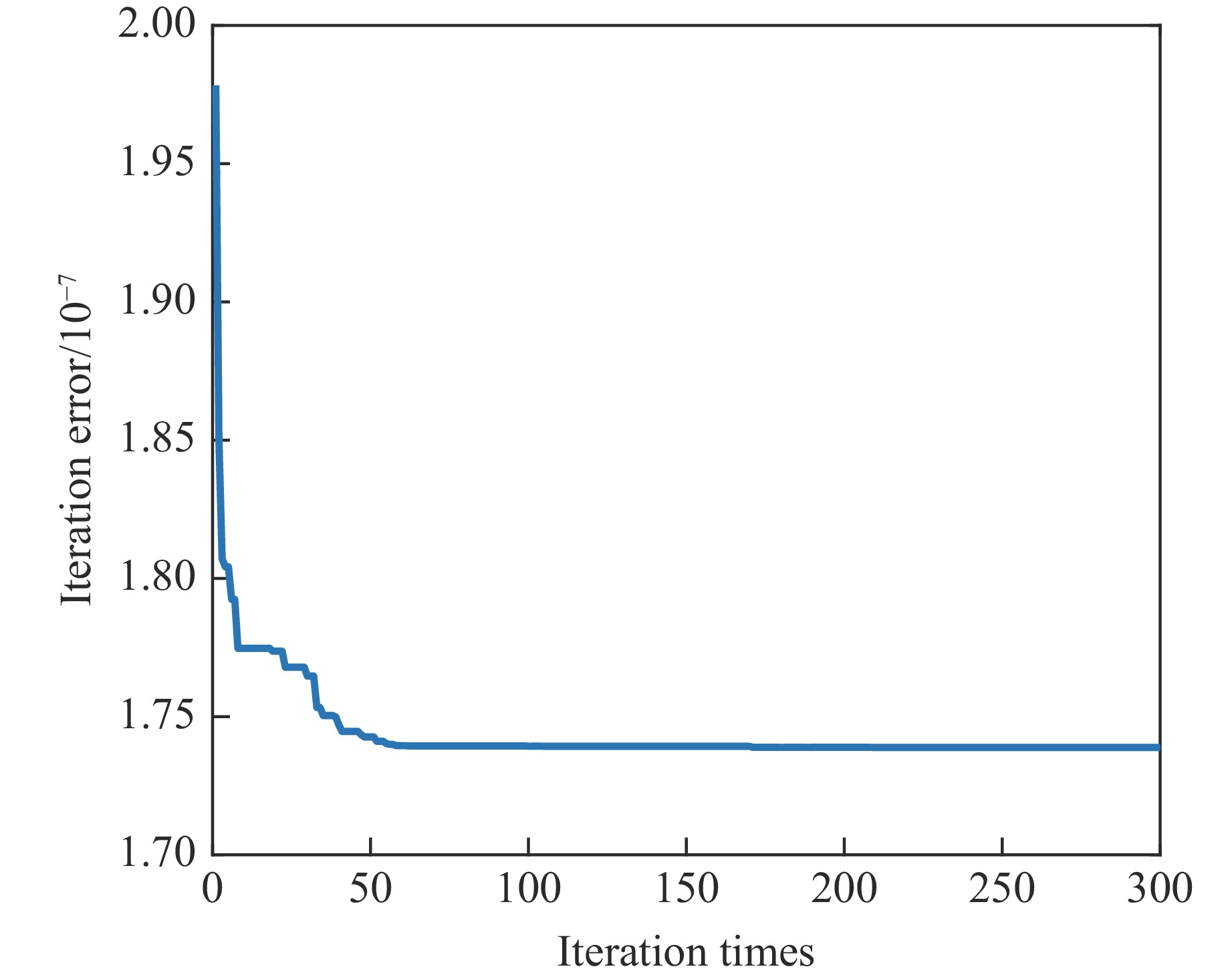
Study and Preliminary Application of the Thermochemical Equation of State of C3N4
-
摘要: C3N4在超硬材料合成和光催化等领域具有广泛的应用,然而,其在高温高压下的相变和物理行为尚未完全清楚,研究其热化学状态方程十分必要。利用分解相边界及常温压缩线数据,提出了一种定量研究C3N4热化学状态方程的高精度、低成本的新方法。对C3N4的石墨相和正交相建立了三项式热化学状态方程,由此计算的诸多物理量与第一性原理计算结果及实验结果吻合良好,证明了热化学状态方程的可靠性。利用C3N4热化学状态方程,对特定温度压力下C3N4的争议相进行了初步判断。此外,将C3N4热化学状态方程加入新型富氮炸药5,5′-联四唑-1,1′-二氧二羟铵(TKX-50)的爆轰参数计算中,显著降低了TKX-50爆轰参数计算值与实验值之间的误差,为新型炸药爆轰机理研究提供了新的参考方向。Abstract: C3N4 has a wide range of applications in the synthesis of superhard materials and photocatalysis materials, but its phase transitions and physical behaviors under high pressure and high temperature conditions are not fully understood. Therefore, it is necessary to study its thermochemical equation of state. In this paper, we propose a novel, high-precision and low-cost method for quantitatively determining the equation of state of C3N4, based on decomposition phase boundary and compression line at room temperature. We constructs the equation of state for two phases of C3N4, and the corresponding physical quantities match well with first-principles calculations and experimental values, proving the reliability of the equation of state. Based on the equation of state of C3N4, we make a preliminary judgment on the phase state of the controversial points. Furthermore, this study attempts to incorporate the equation of state of C3N4 into the research on the detonation mechanism of novel nitrogen-rich explosives. It significantly reduces the long-standing errors between the calculated values and experimental values of the detonation parameters of the explosives, and provides a new reference direction for the research on the detonation parameter calculations of new explosives.
-
Key words:
- C3N4 /
- thermochemical equation of state /
- phase boundary /
- nitrogen-rich explosive
-
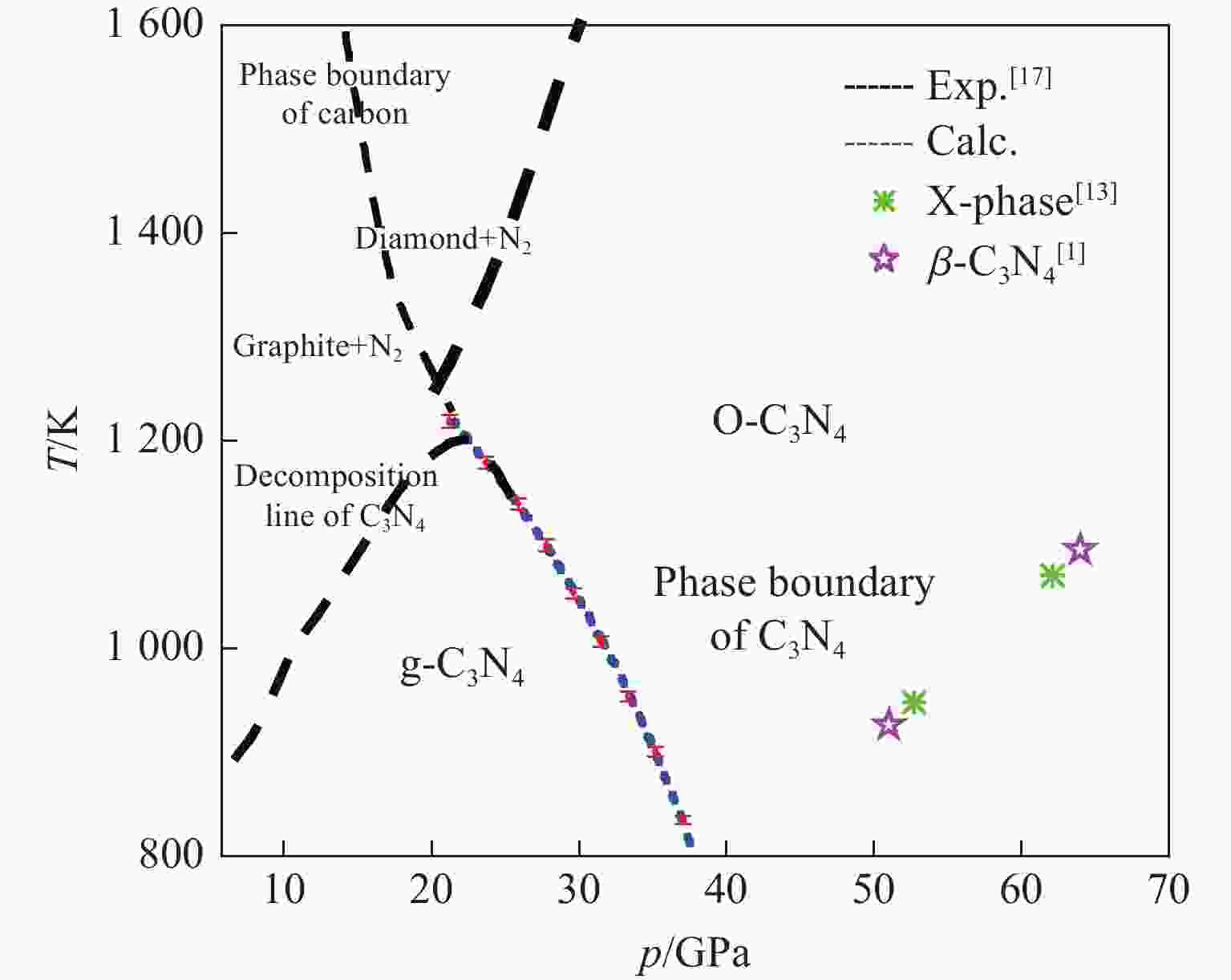
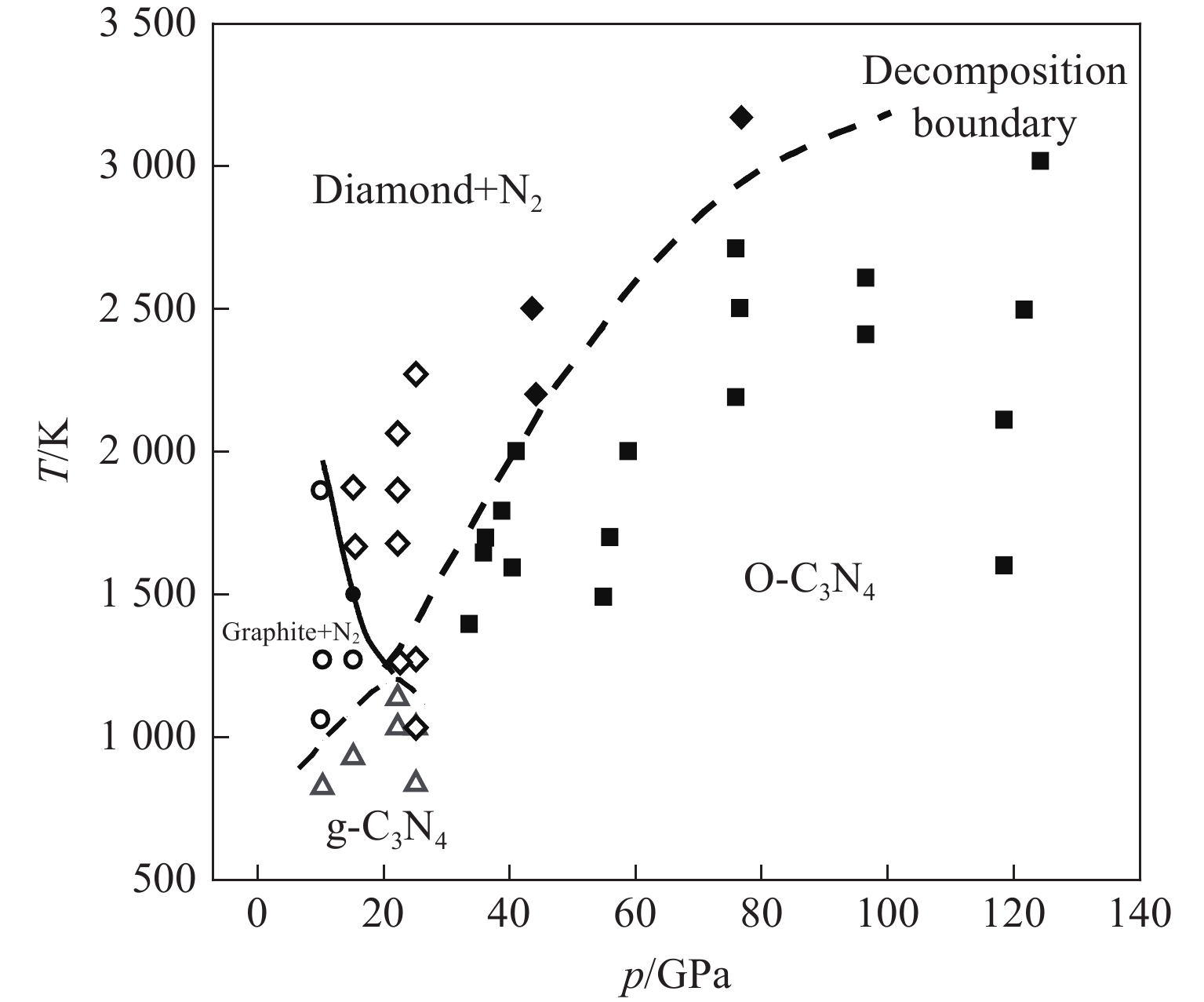
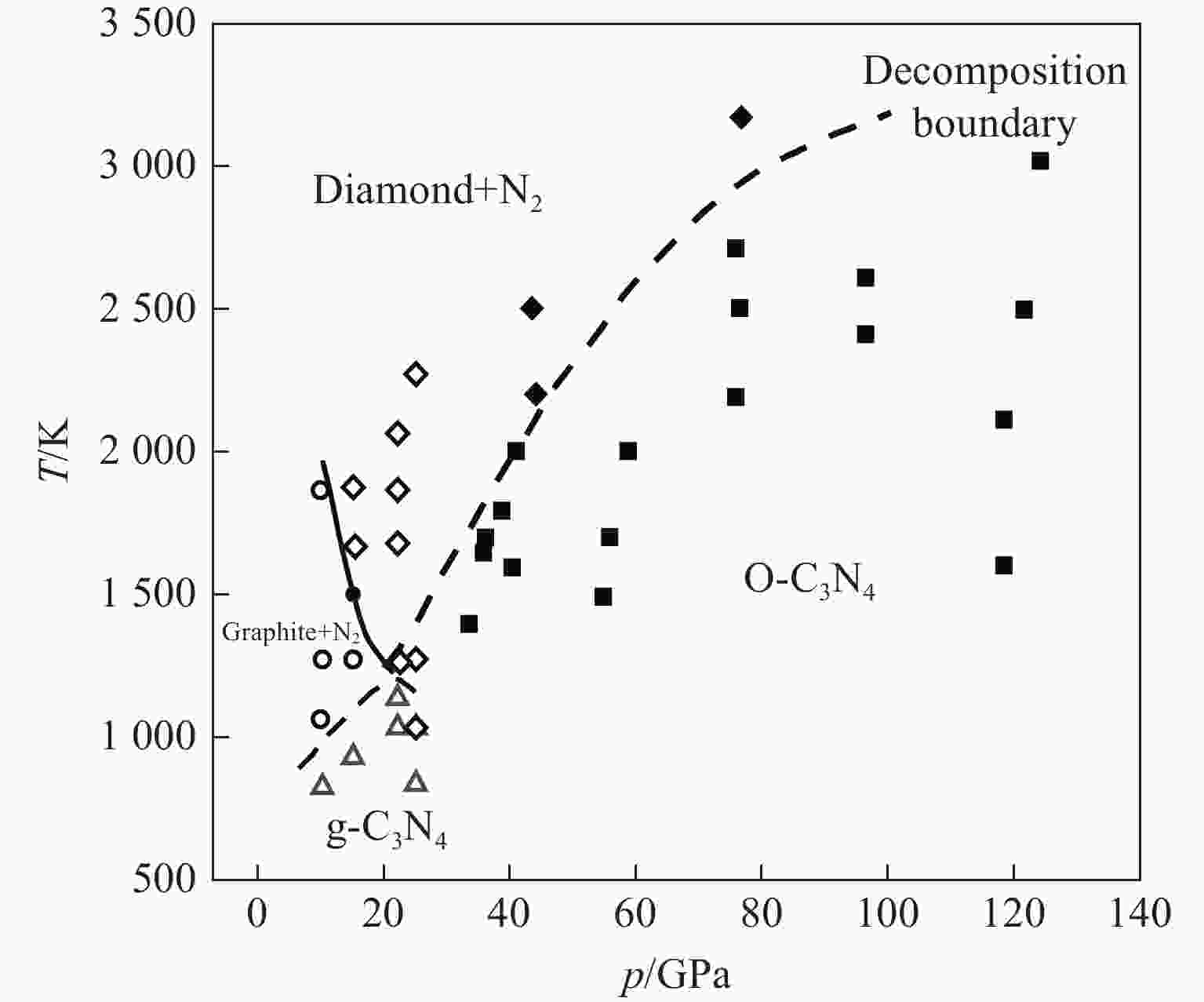
图 1 C3N4的相图[17](圆形、正方形、菱形和三角形分别表示石墨、O-C3N4、金刚石和g-C3N4,实线表示石墨与金刚石的边界,虚线表示C3N4的分解相边界)
Figure 1. Phase diagram of C3N4[17] (Circles, squares, diamonds and triangles denote graphite, O-C3N4, diamond and g-C3N4, respectively. Thick solid line represents the boundary between graphite and diamond, and the dashed line indicates the decomposed phase boundary of C3N4.)
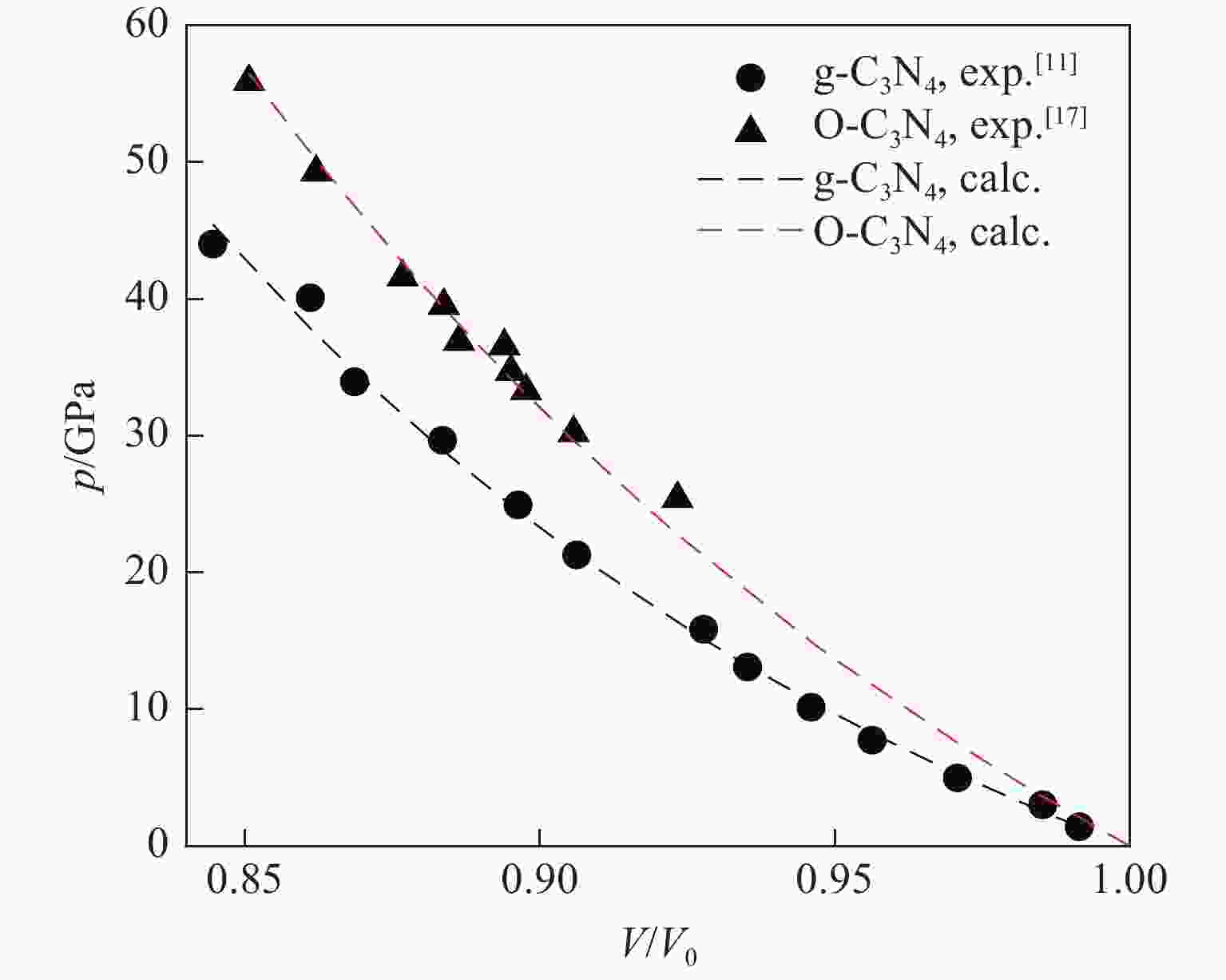
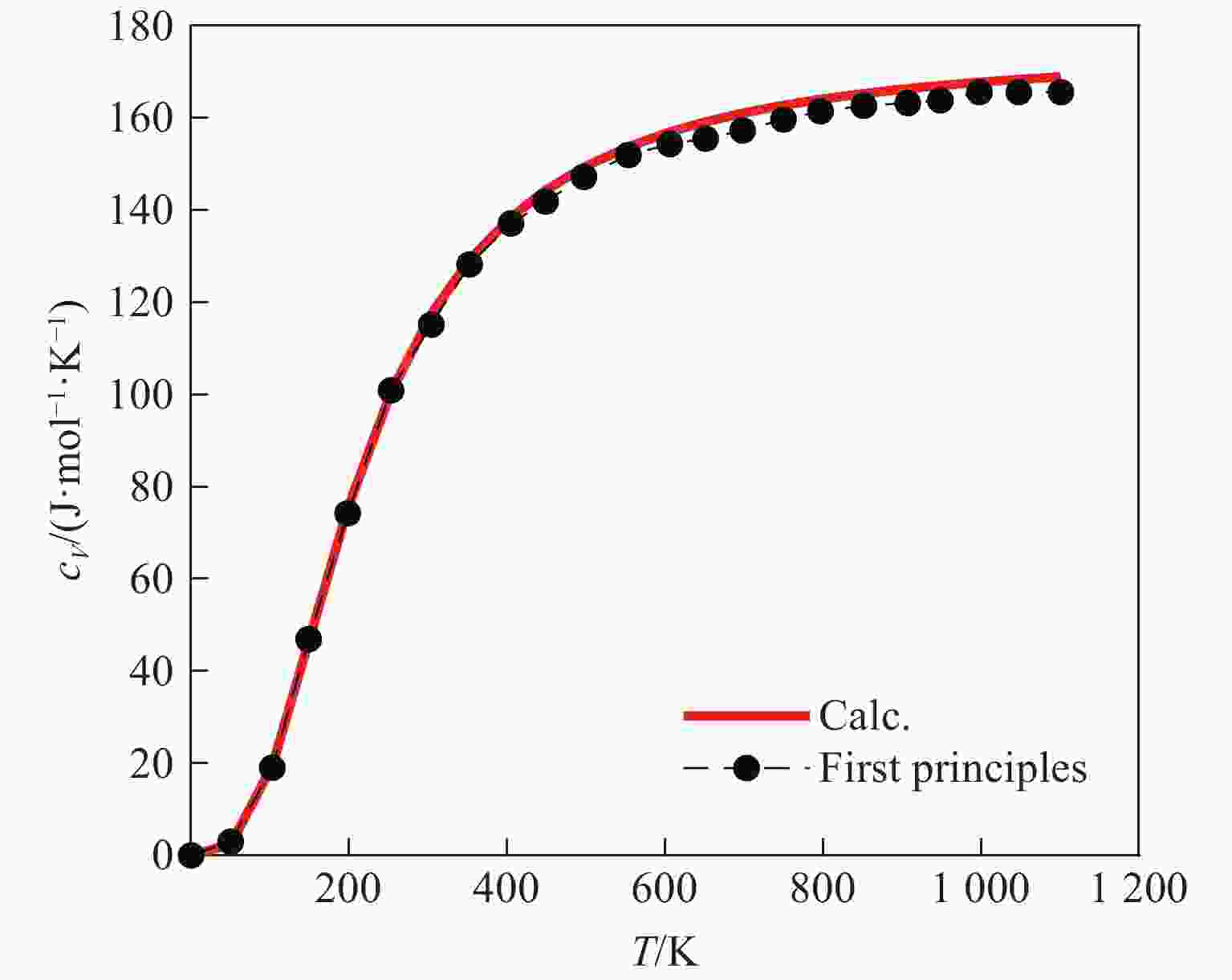
表 1 g-C3N4和O-C3N4的热化学状态方程参数
Table 1. Thermochemical equation of state parameters of g-C3N4 and O-C3N4
Material B0/GPa B1 $ {V_{{\text{0\,K}}}} $/(cm3·g−1) Eref/(kJ·mol−1) Sref/(kJ·mol−1·K−1) c1/(mm·s−1) c2/(mm·s−1) α/(cm3·g−1·K−1) g-C3N4 163.62 5.40 0.449 89.86 3.40×10−2 1.39 −1.07 4.41×10−6 O-C3N4 244.12 4.08 0.401 111.85 8.76×10−2 1.15 −0.47 4.19×10−6 表 2 实验和计算得到TKX-50炸药的CJ爆轰参数
Table 2. CJ parameters of TKX-50 explosives obtained by experiments and calculations
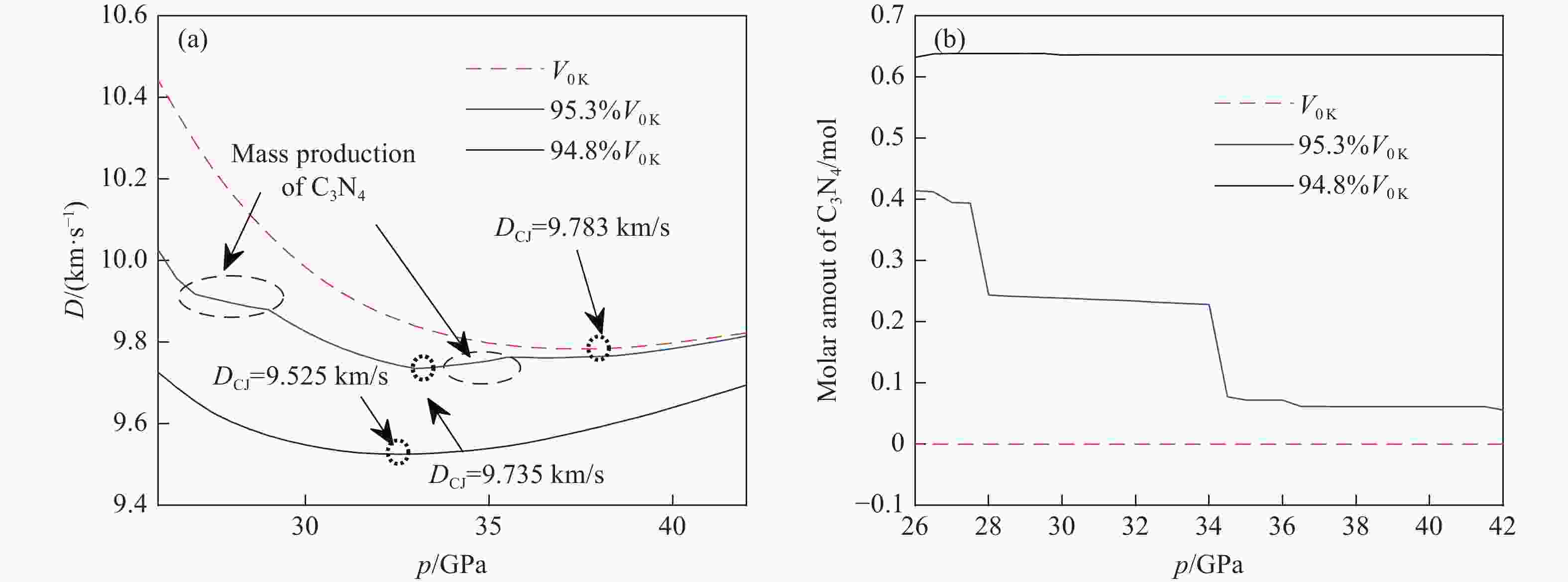
Method ρ0/(g·cm−3) DCJ/(km·s−1) pCJ/GPa TCJ/K Exp. (detonation test)[48] 1.877 9.432 Exp. (damage test)[49] 1.86 9.037 26.40 Exp. (DRZ test)[50] 1.85 9.050 35.04 Calc. ($ {V_{{\text{0\,K}}}} $) 1.85 9.783 38.20 3161.9 Calc. (95.3%$ {V_{{\text{0\,K}}}} $) 1.85 9.735 33.30 3030.9 Calc. (94.8%$ {V_{{\text{0\,K}}}} $) 1.85 9.525 32.50 2953.9 Calc. (Explo 5.05)[44] 1.877 9.698 42.40 3954 Calc. (CHEETAH 8.0)[48] 1.877 9.735 42.40 2845 Calc. (empirical code)[51] 1.877 9.650 41.90 3724 -
[1] WANG Y G, LIU F S, LIU Q J, et al. Recover of C3N4 nanoparticles under high-pressure by shock wave loading [J]. Ceramics International, 2018, 44(16): 19290–19294. doi: 10.1016/j.ceramint.2018.07.155 [2] LANIEL D, TRYBEL F, ZHOU W J, et al. High-pressure synthesis of oP28-C3N4 recoverable to ambient conditions [J]. Advanced Functional Materials, 2025, 35(11): 2416892. doi: 10.1002/ADFM.202416892 [3] SONG X L, CHEN L, GAO L J, et al. Engineering g-C3N4 based materials for advanced photocatalysis: recent advances [J]. Green Energy & Environment, 2024, 9(2): 166–197. doi: 10.1016/j.gee.2022.12.005 [4] MUHMOOD T, AHMAD I, HAIDER Z, et al. Graphene-like graphitic carbon nitride (g-C3N4) as a semiconductor photocatalyst: properties, classification, and defects engineering approaches [J]. Materials Today Sustainability, 2024, 25: 100633. doi: 10.1016/j.mtsust.2023.100633 [5] YU X N, NG S F, PUTRI L K, et al. Point-defect engineering: leveraging imperfections in graphitic carbon nitride (g-C3N4) photocatalysts toward artificial photosynthesis [J]. Small, 2021, 17(48): 2006851. doi: 10.1002/smll.202006851 [6] KLAPÖTKE T M. TKX-50: a highly promising secondary explosive [M]//TRACHE D, BENALIOUCHE F, MEKKI A. Materials Research and Applications: Select Papers from JCH8–2019. Singapore: Springer, 2021: 1–91. [7] WANG X H, HAO G Z, XIAO L, et al. Review on the thermal decomposition of dihydroxylammonium 5, 5′-bistetrazole-1, 1′-diolate (TKX-50) [J]. Thermochimica Acta, 2023, 719: 179393. doi: 10.1016/J.TCA.2022.179393 [8] ZHAO C D, CHI Y, PENG Q, et al. A study on the comprehension of differences in specific kinetic energy of TKX-50 and HMX from the perspective of gas products [J]. Physical Chemistry Chemical Physics, 2019, 21(12): 6600–6605. doi: 10.1039/C8CP07487A [9] REN X, HE R N, WANG X H, et al. A comprehensive experimental and theoretical study of thermal response mechanisms of TKX-50 and HMX [J]. Fuel, 2024, 375: 132623. doi: 10.1016/j.fuel.2024.132623 [10] HAN Y H, LUO J F, GAO C X, et al. Phase transition of graphitic-C3N4 under high pressure by in situ resistance measurement in a diamond anvil cell [J]. Chinese Physics Letters, 2005, 22(6): 1347–1349. doi: 10.1088/0256-307X/22/6/014 [11] 李雪飞, 张剑, 刘伟, 等. 氮化碳的高压同步辐射研究 [J]. 高压物理学报, 2009, 23(1): 71–74. doi: 10.11858/gywlxb.2009.01.012LI X F, ZHANG J, LIU W, et al. Synchrotron radiation X-ray diffraction of carbon nitride under high pressure [J]. Chinese Journal of High Pressure Physics, 2009, 23(1): 71–74. doi: 10.11858/gywlxb.2009.01.012 [12] 李雪飞, 马艳梅, 沈龙海, 等. 石墨相C3N4压致结构相变研究 [J]. 高压物理学报, 2010, 24(1): 67–70. doi: 10.11858/gywlxb.2010.01.012LI X F, MA Y M, SHEN L H, et al. Study on the pressure-induced phase transition of g-C3N4 [J]. Chinese Journal of High Pressure Physics, 2010, 24(1): 67–70. doi: 10.11858/gywlxb.2010.01.012 [13] GAO X, YIN H, CHEN P W, et al. Shock-induced phase transition of g-C3N4 to a new C3N4 phase [J]. Journal of Applied Physics, 2019, 126(15): 155901. doi: 10.1063/1.5111710 [14] 马海云, 刘福生, 李永宏, 等. 强冲击压缩条件下g-C3N4向β-C3N4直接转化 [J]. 高压物理学报, 2012, 26(3): 319–324. doi: 10.11858/gywlxb.2012.03.012MA H Y, LIU F S, LI Y H, et al. Strong shock-compression of g-C3N4 precursor for direct synthesis of β-C3N4 [J]. Chinese Journal of High Pressure Physics, 2012, 26(3): 319–324. doi: 10.11858/gywlxb.2012.03.012 [15] TETER D M, HEMLEY R J. Low-compressibility carbon nitrides [J]. Science, 1996, 271(5245): 53–55. doi: 10.1126/science.271.5245.53 [16] LIU A Y, COHEN M L. Prediction of new low compressibility solids [J]. Science, 1989, 245(4920): 841–842. doi: 10.1126/science.245.4920.841 [17] KOJIMA Y, OHFUJI H. Structure and stability of carbon nitride under high pressure and high temperature up to 125 GPa and 3000 K [J]. Diamond and Related Materials, 2013, 39: 1–7. doi: 10.1016/j.diamond.2013.07.006[18] MING L C, ZININ P, MENG Y, et al. A cubic phase of C3N4 synthesized in the diamond-anvil cell [J]. Journal of Applied Physics, 2006, 99(3): 033520. doi: 10.1063/1.2168567 [19] FANG L M, OHFUJI H, SHINMEI T, et al. Experimental study on the stability of graphitic C3N4 under high pressure and high temperature [J]. Diamond and Related Materials, 2011, 20(5/6): 819–825. doi: 10.1016/j.diamond.2011.03.034 [20] 邹广田, 李雪飞, 杨大鹏, 等. 石墨相C3N4的高温高压研究 [J]. 原子与分子物理学报, 2009, 26(4): 705–707. doi: 10.3969/j.issn.1000-0364.2009.04.024ZOU G T, LI X F, YANG D P, et al. Study on the pressure-induced phase transition of g-C3N4 [J]. Journal of Atomic and Molecular Physics, 2009, 26(4): 705–707. doi: 10.3969/j.issn.1000-0364.2009.04.024 [21] LANIEL D, TRYBEL F, ASLANDUKOV A, et al. Synthesis of ultra-incompressible and recoverable carbon nitrides featuring CN4 tetrahedra [J]. Advanced Materials, 2024, 36(3): 2308030. doi: 10.1002/adma.202308030 [22] MANYALI G S, WARMBIER R, QUANDT A, et al. Ab initio study of elastic properties of super hard and graphitic structures of C3N4 [J]. Computational Materials Science, 2013, 69: 299–303. doi: 10.1016/j.commatsci.2012.11.039 [23] LANZILOTTO V, SILVA J L, ZHANG T, et al. Spectroscopic fingerprints of intermolecular H-bonding interactions in carbon nitride model compounds [J]. Chemistry–A European Journal, 2018, 24(53): 14198–14206. doi: 10.1002/chem.201802435 [24] UGOLOTTI A, DI VALENTIN C. Ab-initio spectroscopic characterization of melem-based graphitic carbon nitride polymorphs [J]. Nanomaterials, 2021, 11(7): 1863. doi: 10.3390/nano11071863 [25] 阮林伟. g-C3N4光催化材料的第一性原理研究 [D]. 合肥: 安徽大学, 2015: 42–56.RUAN L W. First-principles study of g-C3N4 photocatalytic materials [D]. Hefei: Anhui University, 2015: 42–56. [26] ZHAO Y R, ZHANG H R, ZHANG G T, et al. First-principles investigation on elastic and thermodynamic properties of Pnnm-CN under high pressure [J]. AIP Advances, 2016, 6(12): 125040. doi: 10.1063/1.4972775 [27] RUAN L W, ZHU Y J, QIU L G, et al. First principles calculations of the pressure affection to g-C3N4 [J]. Computational Materials Science, 2014, 91: 258–265. doi: 10.1016/j.commatsci.2014.04.058 [28] PRIBYLOV A A, POSTNIKOV E B. Thermodynamic curvature and the thermal expansion isolines [J]. Journal of Molecular Liquids, 2021, 335: 115994. doi: 10.1016/j.molliq.2021.115994 [29] RIBEIRO M, HENRIQUES T, CASTRO L, et al. The entropy universe [J]. Entropy, 2021, 23(2): 222. [30] BLANCO M A, FRANCISCO E, LUAÑA V. GIBBS: isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model [J]. Computer Physics Communications, 2004, 158(1): 57–72. doi: 10.1016/j.comphy.2003.12.001 [31] LUO Y F, LI M K, YUAN H M, et al. Predicting lattice thermal conductivity via machine learning: a mini review [J]. NPJ Computational Materials, 2023, 9(1): 4. doi: 10.1038/s41524-023-00964-2 [32] XIAN Y T, XIANG S K, LIU L, et al. Accurate equation of state of rhenium as pressure scale up to 130 GPa and 3200 K [J]. AIP Advances, 2022, 12(5): 055313. doi: 10.1063/5.0089292[33] LUO Y, XIANG S K, LI J, et al. Equation of state of MgO up to 345 GPa and 8500 K [J]. Physical Review B, 2023, 107(13): 134116. doi: 10.1103/PhysRevB.107.134116[34] KRUKOWSKI S, STRĄK P. Equation of state of nitrogen (N2) at high pressures and high temperatures: molecular dynamics simulation [J]. The Journal of Chemical Physics, 2006, 124(13): 134501. doi: 10.1063/1.2185096 [35] 杨金文, 施尚春, 李巧燕, 等. 高温高密度液氦冲击压缩特性理论研究 [J]. 爆炸与冲击, 2007, 27(6): 557–561. doi: 10.11883/1001-1455(2007)06-0557-05YANG J W, SHI S C, LI Q Y, et al. Theoretical research on shock compression properties of liquid helium at high temperature and density [J]. Explosion and Shock Waves, 2007, 27(6): 557–561. doi: 10.11883/1001-1455(2007)06-0557-05 [36] 赵艳红. 基于统计物理和化学平衡的爆轰产物物态方程 [D]. 绵阳: 中国工程物理研究院, 2015: 21–46.ZHAO Y H. The equation of state of detonation products based on statistical physics and chemical equilibrium [D]. Mianyang: China Academy of Engineering Physics, 2015: 21–46. [37] FRIED L E, HOWARD W M. Explicit Gibbs free energy equation of state applied to the carbon phase diagram [J]. Physical Review B, 2000, 61(13): 8734–8743. doi: 10.1103/PhysRevB.61.8734 [38] JAWORSKI Z, ZAKRZEWSKA B, PIANKO-OPRYCH P. On thermodynamic equilibrium of carbon deposition from gaseous C-H-O mixtures: updating for nanotubes [J]. Reviews in Chemical Engineering, 2017, 33(3): 217–235. doi: 10.1515/revce-2016-0022 [39] 王中友, 李星翰, 甘云丹, 等. 爆热弹中产物组分演化的计算研究 [J]. 火炸药学报, 2022, 45(2): 229–242. doi: 10.14077/j.issn.1007-7812.202112008WANG Z Y, LI X H, GAN Y D, et al. Study on thermodynamic evolution of detonation products in the detonation bomb test [J]. Chinese Journal of Explosives & Propellants, 2022, 45(2): 229–242. doi: 10.14077/j.issn.1007-7812.202112008 [40] LI X H, YI Z C, LIU Q J, et al. Research of detonation products of RDX/Al from the perspective of composition [J]. Defence Technology, 2023, 24: 31–45. doi: 10.1016/j.dt.2022.11.016 [41] KATOCH S, CHAUHAN S S, KUMAR V. A review on genetic algorithm: past, present, and future [J]. Multimedia Tools and Applications, 2021, 80(5): 8091–8126. doi: 10.1007/s11042-020-10139-6 [42] WANG D S, TAN D P, LIU L. Particle swarm optimization algorithm: an overview [J]. Soft Computing, 2018, 22(2): 387–408. doi: 10.1007/s00500-016-2474-6 [43] CANG Y P, LIAN S B, YANG H M, et al. Predicting physical properties of tetragonal, monoclinic and orthorhombic M3N4 (M= C, Si, Sn) polymorphs via first-principles calculations [J]. Chinese Physics Letters, 2016, 33(6): 066301. doi: 10.1088/0256-307X/33/6/066301 [44] FISCHER N, FISCHER D, KLAPÖTKE T M, et al. Pushing the limits of energetic materials: the synthesis and characterization of dihydroxylammonium 5, 5′-bistetrazole-1, 1′-diolate [J]. Journal of Materials Chemistry, 2012, 22(38): 20418–20422. doi: 10.1039/C2JM33646D [45] SUCESKA M, TUMARA B S, DOBRILOVIC M, et al. Estimation of detonation front curvature radius by empirical equations [J]. Journal of Energetic Materials, 2024, 42(2): 169–186. doi: 10.1080/07370652.2022.2052207 [46] SAIF M, WANG W T, PEKALSKI A, et al. Chapman-Jouguet deflagrations and their transition to detonation [J]. Proceedings of the Combustion Institute, 2017, 36(2): 2771–2779. doi: 10.1016/j.proci.2016.07.122 [47] 杨舒棋, 张旭, 彭文杨, 等. 钝感炸药冲击起爆反应过程的PDV技术 [J]. 高压物理学报, 2020, 34(2): 023402. doi: 10.11858/gywlxb.20190856YANG S Q, ZHANG X, PENG W Y, et al. PDV technology of shock initiation reaction process of insensitive explosive [J]. Chinese Journal of High Pressure Physics, 2020, 34(2): 023402. doi: 10.11858/gywlxb.20190856 [48] GOTTFRIED J L, KLAPÖTKE T M, WITKOWSKI T G. Estimated detonation velocities for TKX-50, MAD-X1, BDNAPM, BTNPM, TKX-55, and DAAF using the laser-induced air shock from energetic materials technique [J]. Propellants, Explosives, Pyrotechnics, 2017, 42(4): 353–359. doi: 10.1002/prep.201600257 [49] 刘佳辉, 范桂娟, 卢校军, 等. TKX-50基混合炸药的爆轰及安全性能 [J]. 含能材料, 2019, 27(11): 902–907. doi: 10.11943/CJEM2019052LIU J H, FAN G J, LU X J, et al. Detonation and safety performance of TKX-50 based PBX [J]. Chinese Journal of Energetic Materials, 2019, 27(11): 902–907. doi: 10.11943/CJEM2019052 [50] TAN K Y, HAN Y, LIU J H, et al. Detonation reaction zone and acceleration ability of a TKX-50 based polymer bonded explosive [J]. Propellants, Explosives, Pyrotechnics, 2023, 48(1): e202100367. doi: 10.1002/PREP.202100367 [51] KESHAVARZ M, ABADI Y H, ESMAEILPOUR K, et al. Novel high-nitrogen content energetic compounds with high detonation and combustion performance for use in plastic bonded explosives (PBXs) and composite solid propellants [J]. Central European Journal of Energetic Materials, 2018, 15(2): 364–375. doi: 10.22211/cejem/78091 -






 下载:
下载: