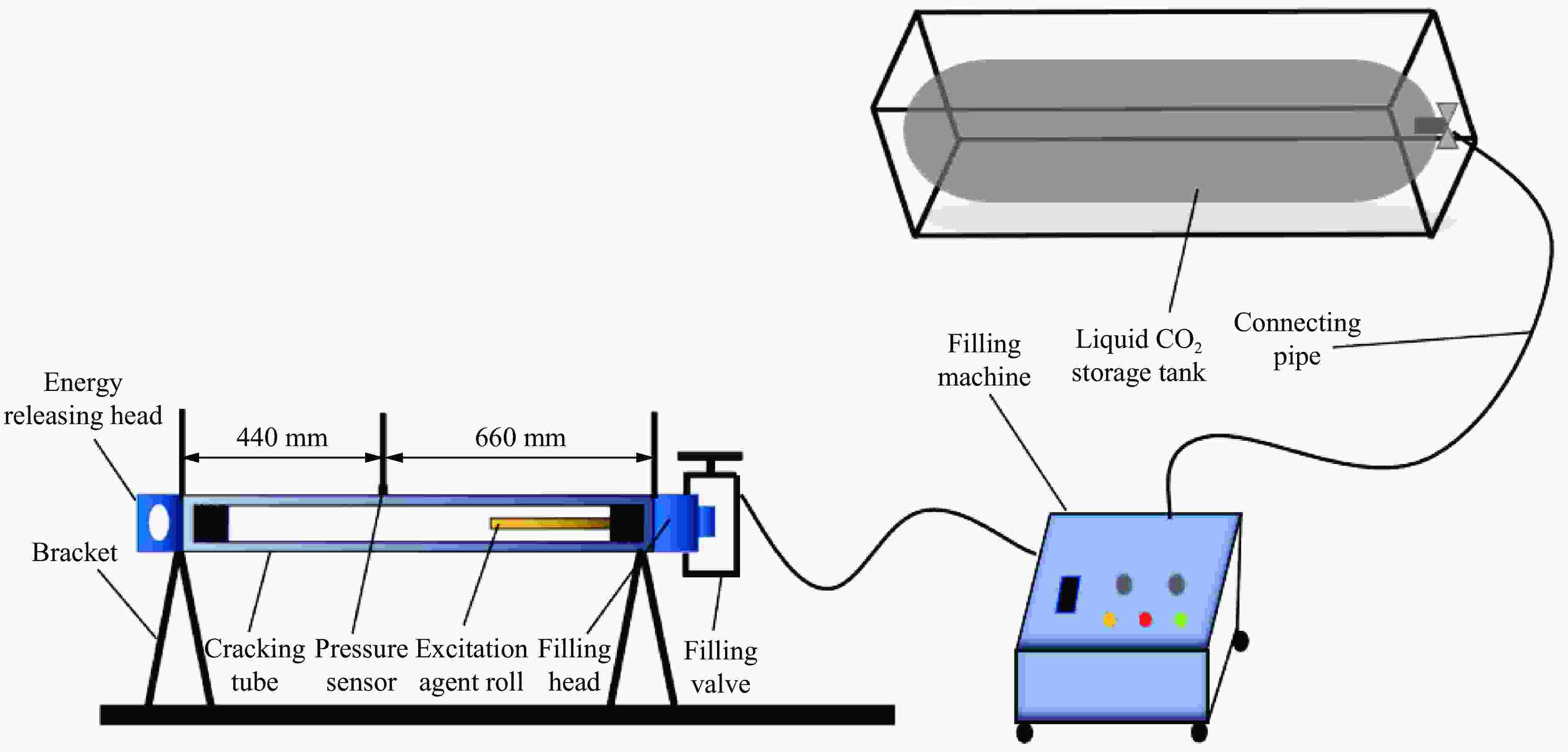
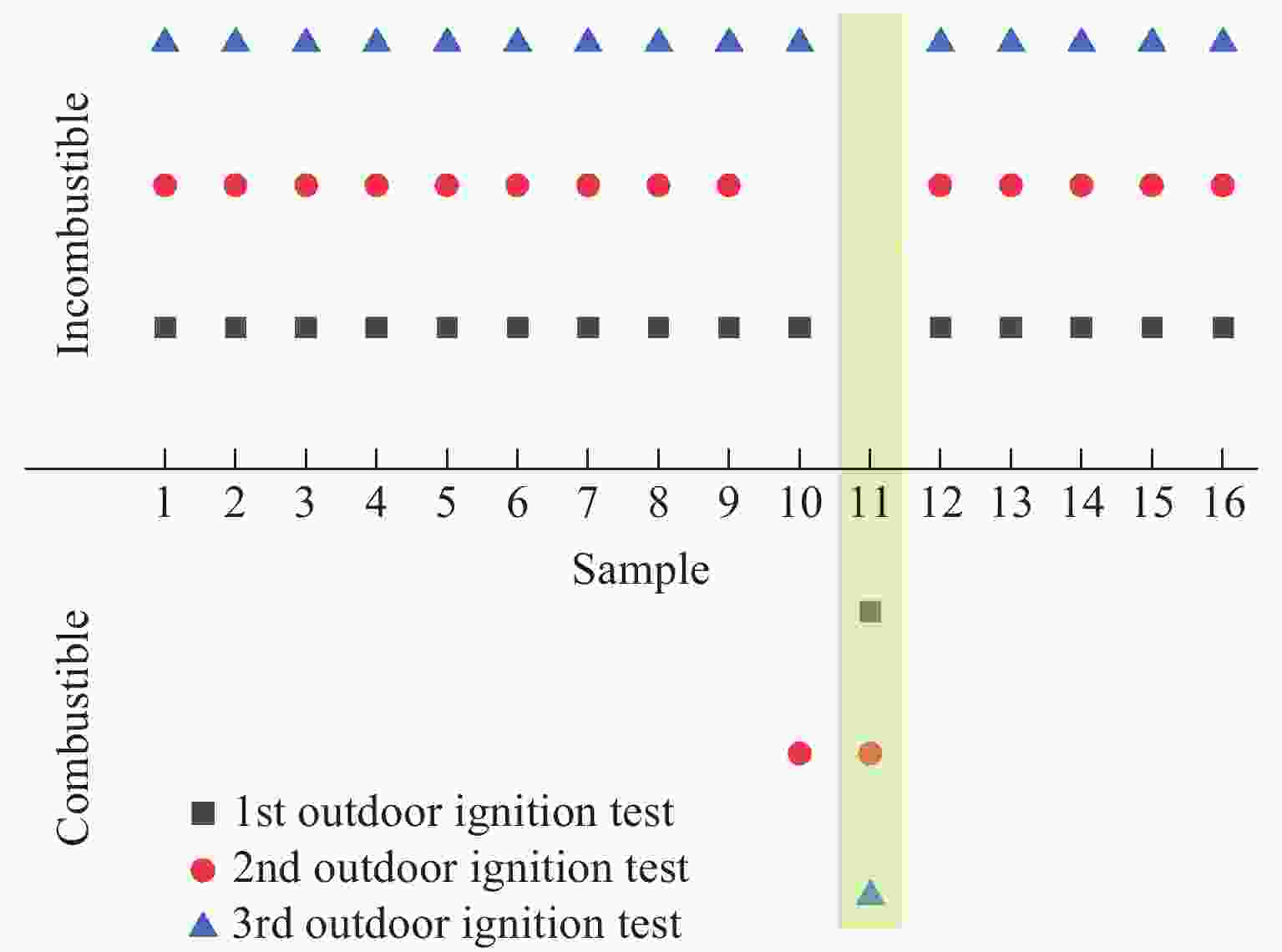
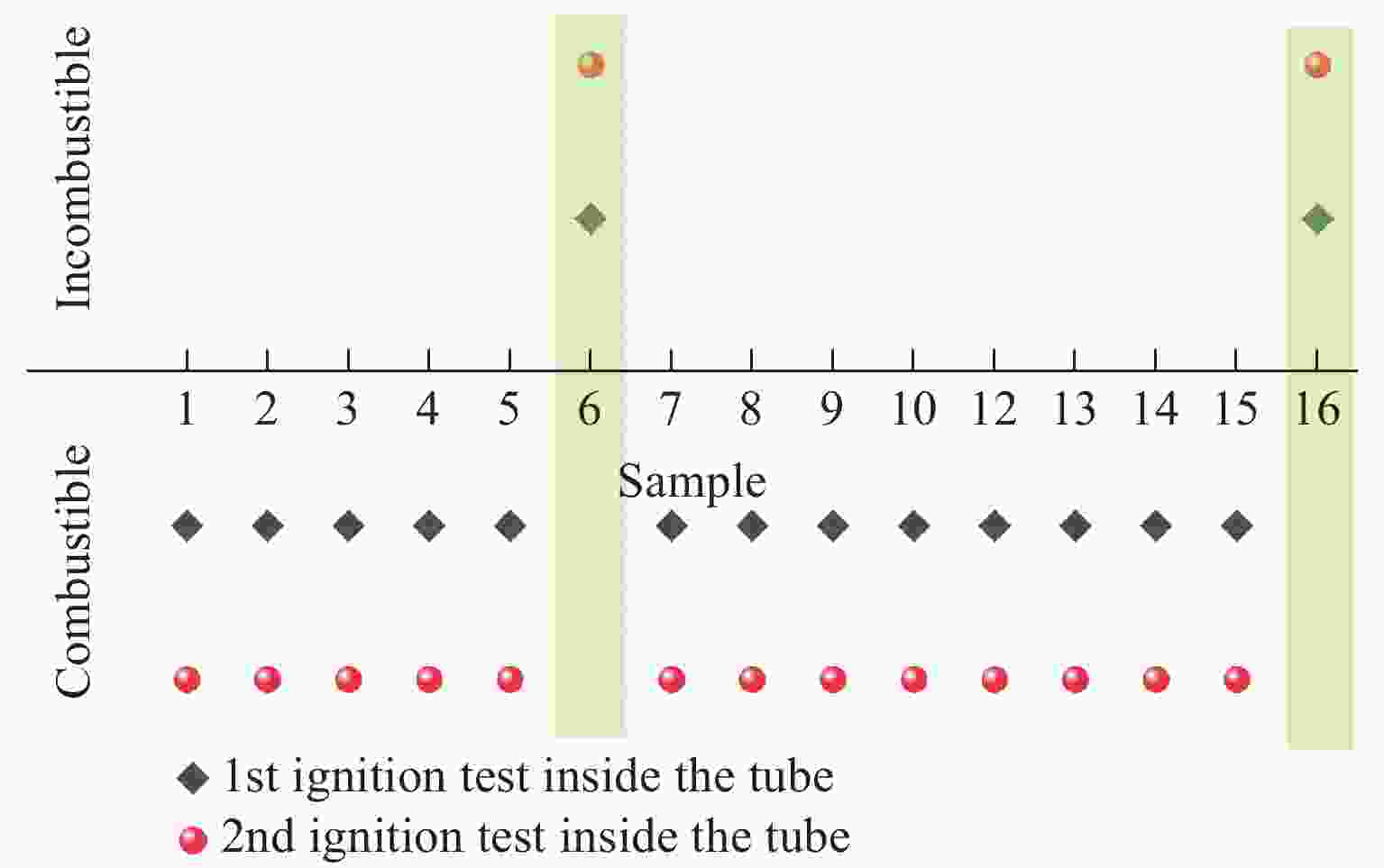
Performance Characterization of CO2 Phase Change Excitation Agent under the Synergistic Effect of Titanium Powder Content and Zero Oxygen Balance
-
摘要: 为了提高CO2相变激发药剂的性能,向激发药剂中分别加入质量分数为2%、4%、6%、8%和10%的钛粉,并调整草酸铵和水杨酸含量,使药剂接近零氧平衡,通过现场引燃试验、热重法、耐温性能测试和理论计算,研究其引燃可靠性、压力性能、热分解特性、安全性能和耐温性能。结果表明:加入质量分数为2%、4%、6%、8%的钛粉后,管内激发药剂均能被成功引燃;峰值压力与药剂放热量直接相关,在本试验的添加范围内,质量分数为8%钛粉激发药剂的管内压力性能最好;添加8%钛粉不调整零氧平衡、通过草酸铵调整零氧平衡、通过水杨酸调整零氧平衡的峰值压力分别提高了11.81%、14.27%、17.85%;3个样品的表观活化能变化分别为−5.96、33.47 和6.80 kJ/mol;调整零氧平衡可以优化激发药剂的热稳定性,加入8%钛粉后激发药剂的安全性良好,温度指数Ts均在90 ℃以上。Abstract: To improve the performance of the CO2 phase change excitation agent, titanium powder with mass fractions of 2%, 4%, 6%, 8%, and 10% was added to the excitation agent. The contents of ammonium oxalate and salicylic acid were controlled to adjust the zero oxygen balance, respectively. The ignition reliability, pressure performance, thermal decomposition characteristics, safety performance and temperature resistance property were investigated by on-site ignition tests, thermogravimetric analysis, temperature resistance performance tests and theoretical calculations. The results show that: all the excitation agents are successfully ignited inside the tube after adding titanium powder with mass fractions of 2%, 4%, 6%, and 8%. The peak pressure is directly associated with the heat release amount of the excitation agent. Within the addition range of this test, the excitation agent with 8% titanium powder has the best pressure performance inside the tube. After adding titanium powder with a mass fraction of 8%, the peak pressures of excitation agent without adjusting the oxygen balance, with adjusting the zero oxygen balance through ammonium oxalate, and with adjusting the zero oxygen balance through salicylic acid increase by 11.81%, 14.27%, and 17.85%, respectively. The apparent activation energies of the three samples decrease by 5.96 kJ/mol, increase by 33.47 and 6.80 kJ/mol, respectively, indicating that adjusting the zero oxygen balance can optimize the thermal stability of the excitation agent. After adding titanium powder with a mass fraction of 8%, the safety of the excitation agent is good, and the temperature index Ts is above 90 ℃.
-
表 1 基础激发药剂样品的配比
Table 1. Basic excitation agent sample formula
Sample Mass/g ωTi/% OB/(g·g−1) KClO4 C2H8N2O4 C7H6O3 Ti 1 82.439 35.366 12.195 0 0 0.00035 2 82.439 35.366 12.195 2.6 1.96 − 0.01272 3 82.439 35.366 12.195 5.2 3.85 − 0.02530 4 82.439 35.366 12.195 7.8 5.66 − 0.03740 5 82.439 35.366 12.195 10.4 7.40 − 0.04905 6 82.439 35.366 12.195 13.0 9.09 − 0.06028 表 2 草酸铵调整零氧平衡样品的配比
Table 2. Sample formula of adjusting zero oxygen balance by the content of ammonium oxalate
Sample Mass/g ωTi/% OB/(g·g−1) KClO4 C2H8N2O4 C7H6O3 Ti 7 82.439 32.076 12.195 2.6 2.01 0.00001 8 82.439 28.712 12.195 5.2 4.05 0.00001 9 82.439 25.352 12.195 7.8 6.10 0.00001 10 82.439 22.005 12.195 10.4 8.19 0.00001 11 82.439 18.661 12.195 13.0 10.29 0.00001 表 3 水杨酸调整零氧平衡样品的配比
Table 3. Sample formula of adjusting zero oxygen balance by the content of salicylic acid
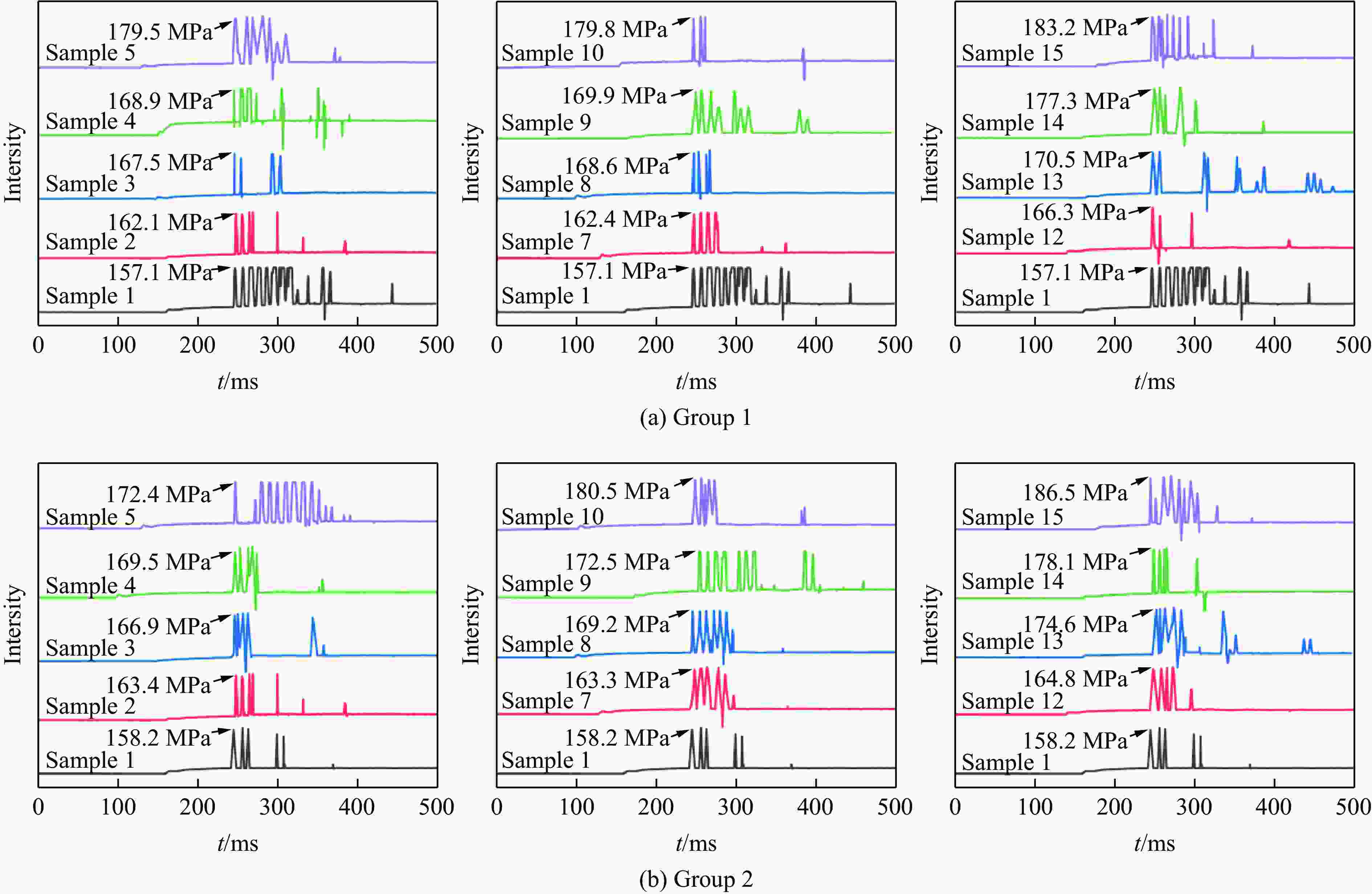
Sample Mass/g ωTi/% OB/(g·g−1) KClO4 C2H8N2O4 C7H6O3 Ti 12 82.439 35.366 11.146 2.6 1.97 0.00001 13 82.439 35.366 10.036 5.2 3.90 0.00001 14 82.439 35.366 9.013 7.8 5.78 0.00001 15 82.439 35.366 7.952 10.4 7.62 0.00001 16 82.439 35.366 6.894 13.0 9.44 0.00001 表 4 试验样品的峰值压力数据
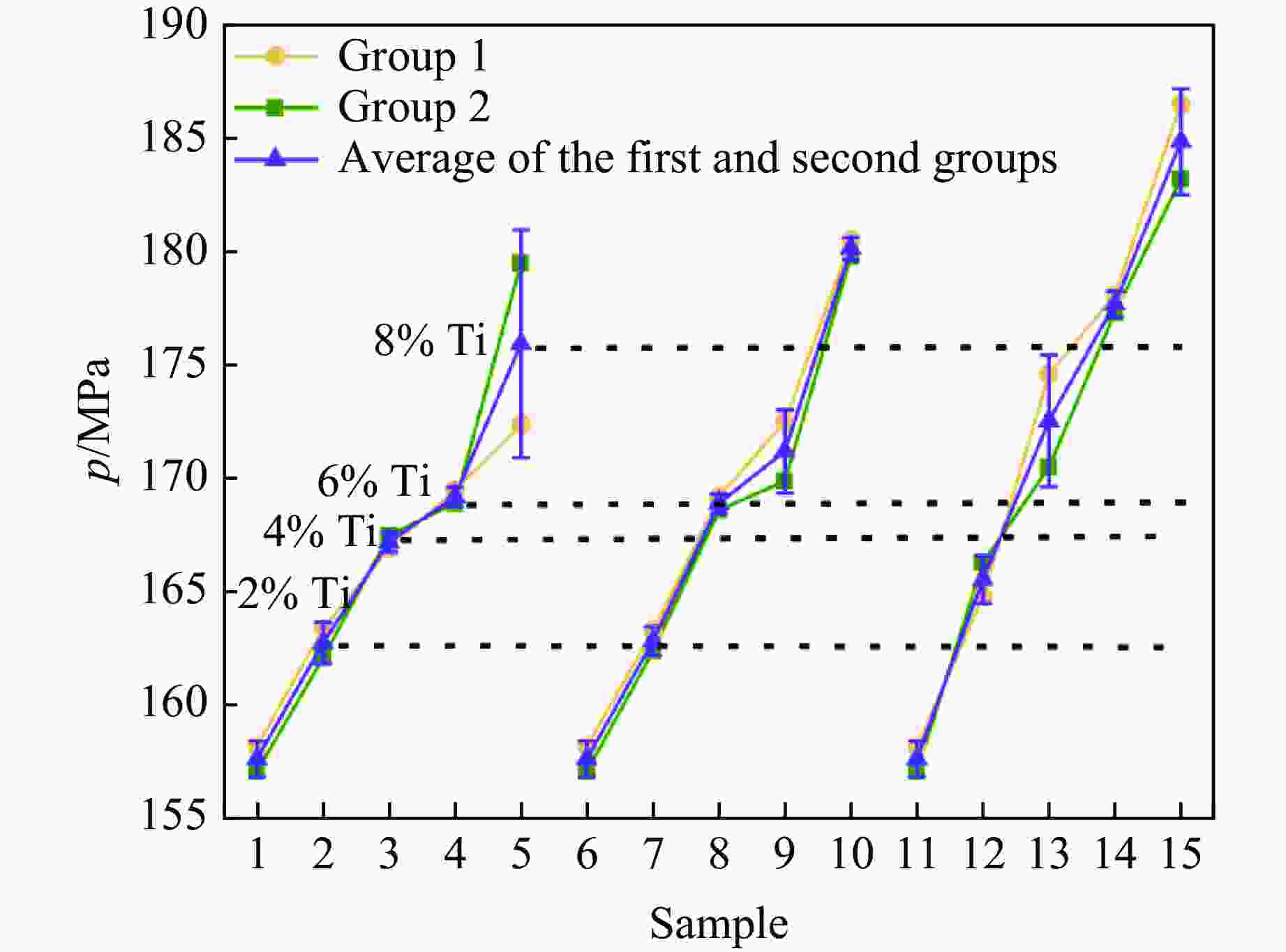
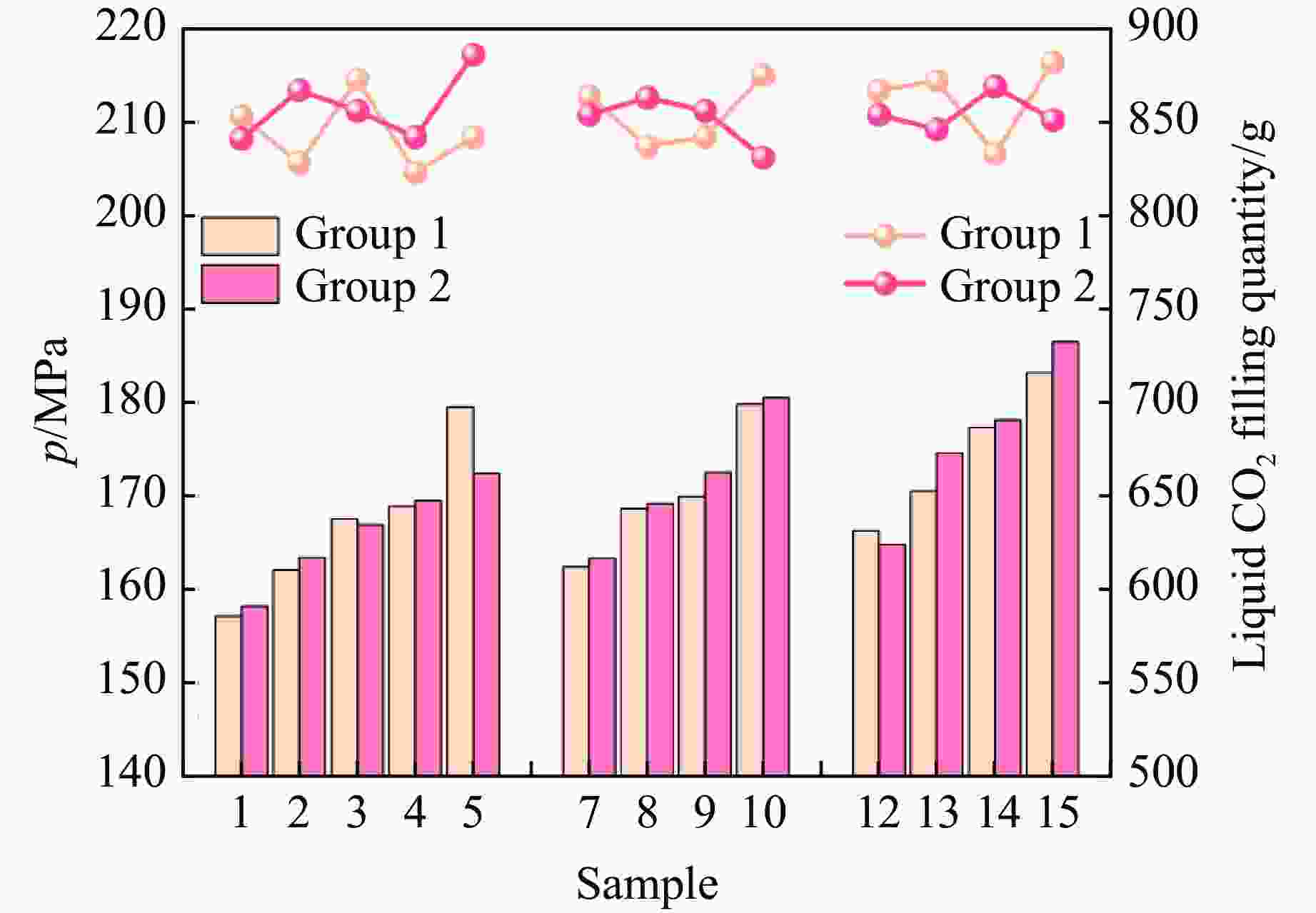
Table 4. Peak pressure data of the test samples
Group Sample Empty tube quality/kg Tube quality after
filling liquid/kgLiquid CO2 charge
amount/gSurge pressure/MPa 1 1 13.856 14.709 853 157.1 2 13.855 14.683 828 162.1 3 13.854 14.727 873 167.5 4 13.856 14.679 823 168.9 5 13.856 14.698 842 179.5 7 13.855 14.719 864 162.4 8 13.854 14.691 837 168.6 9 13.858 14.700 842 169.9 10 13.856 14.731 875 179.8 12 13.854 14.721 867 166.3 13 13.855 14.727 872 170.5 14 13.855 14.718 833 177.3 15 13.855 14.737 882 183.2 2 1 13.858 14.699 841 158.2 2 13.856 14.723 867 163.4 3 13.855 14.711 856 166.9 4 13.857 14.699 842 169.5 5 13.854 14.740 886 172.4 7 13.856 14.710 854 163.3 8 13.855 14.718 863 169.2 9 13.856 14.712 856 172.5 10 13.855 14.686 831 180.5 12 13.857 14.711 854 164.8 13 13.856 14.702 846 174.6 14 13.856 14.695 869 178.1 15 13.854 14.705 851 186.5 表 5 激发药剂反应热理论值
Table 5. Theoretical reaction heat values of excitation agent
Sample ΔHT/(kJ·kg−1) 1 5203.0 2 5261.5 3 5311.8 4 5360.1 5 5406.7 7 5325.7 8 5447.3 9 5570.4 10 5694.3 12 5363.3 13 5516.7 14 5668.2 15 5816.2 表 6 各样品的瞬间压剪速度
Table 6. Instantaneous compression-shear speed of each sample
Sample Pressure rise time/ms Instantaneous compression-shear
speed/(m·s−1)Average instantaneous
compression-shear
speed/(m·s−1)Group 1 Group 2 Group 1 Group 2 1 2.82 5.86 156.03 75.09 115.56 2 3.03 1.72 145.21 255.81 200.51 3 2.61 2.58 168.58 170.54 169.56 4 1.26 3.21 349.21 137.07 243.14 5 4.04 2.92 108.91 150.68 129.80 7 3.06 4.22 143.79 104.27 124.03 8 2.53 2.54 173.91 173.23 173.57 9 4.69 2.11 93.82 208.53 151.17 10 1.54 3.68 285.71 119.57 202.64 12 2.54 5.02 173.23 87.65 130.44 13 3.69 4.29 119.24 102.56 110.90 14 3.66 1.82 120.22 241.76 180.99 15 2.74 1.61 160.58 273.29 216.94 表 7 Ozawa法计算各样品的动力学参数
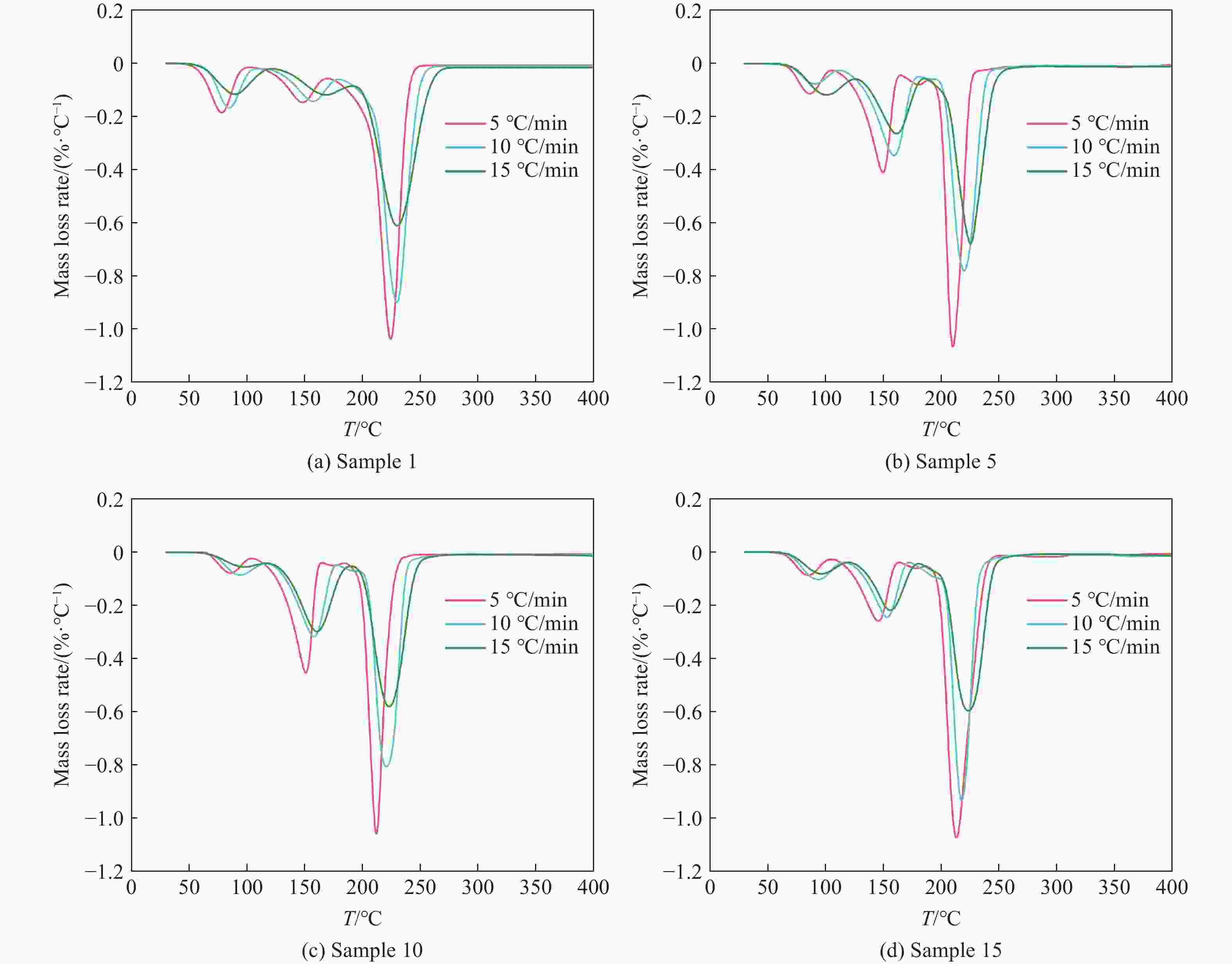
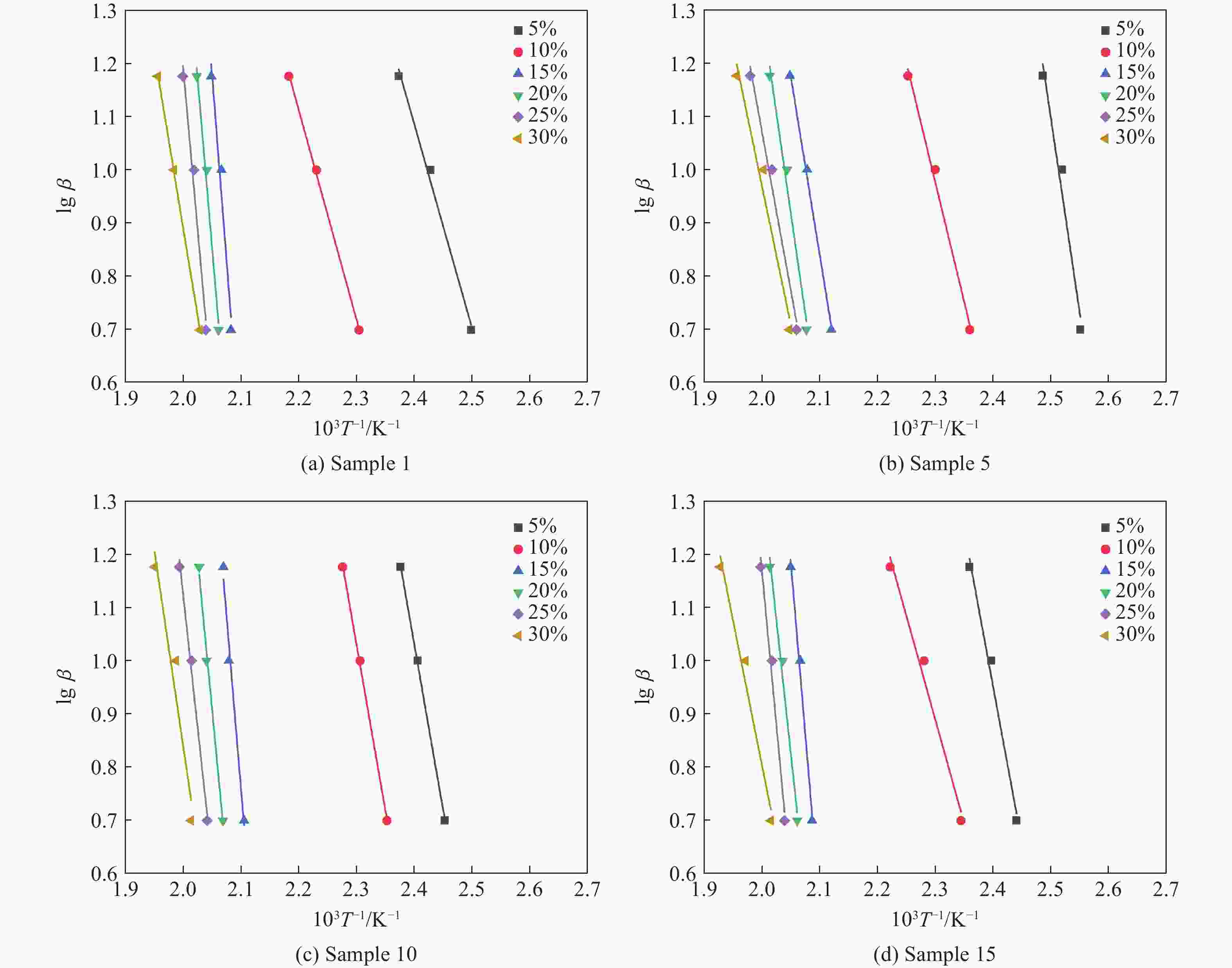
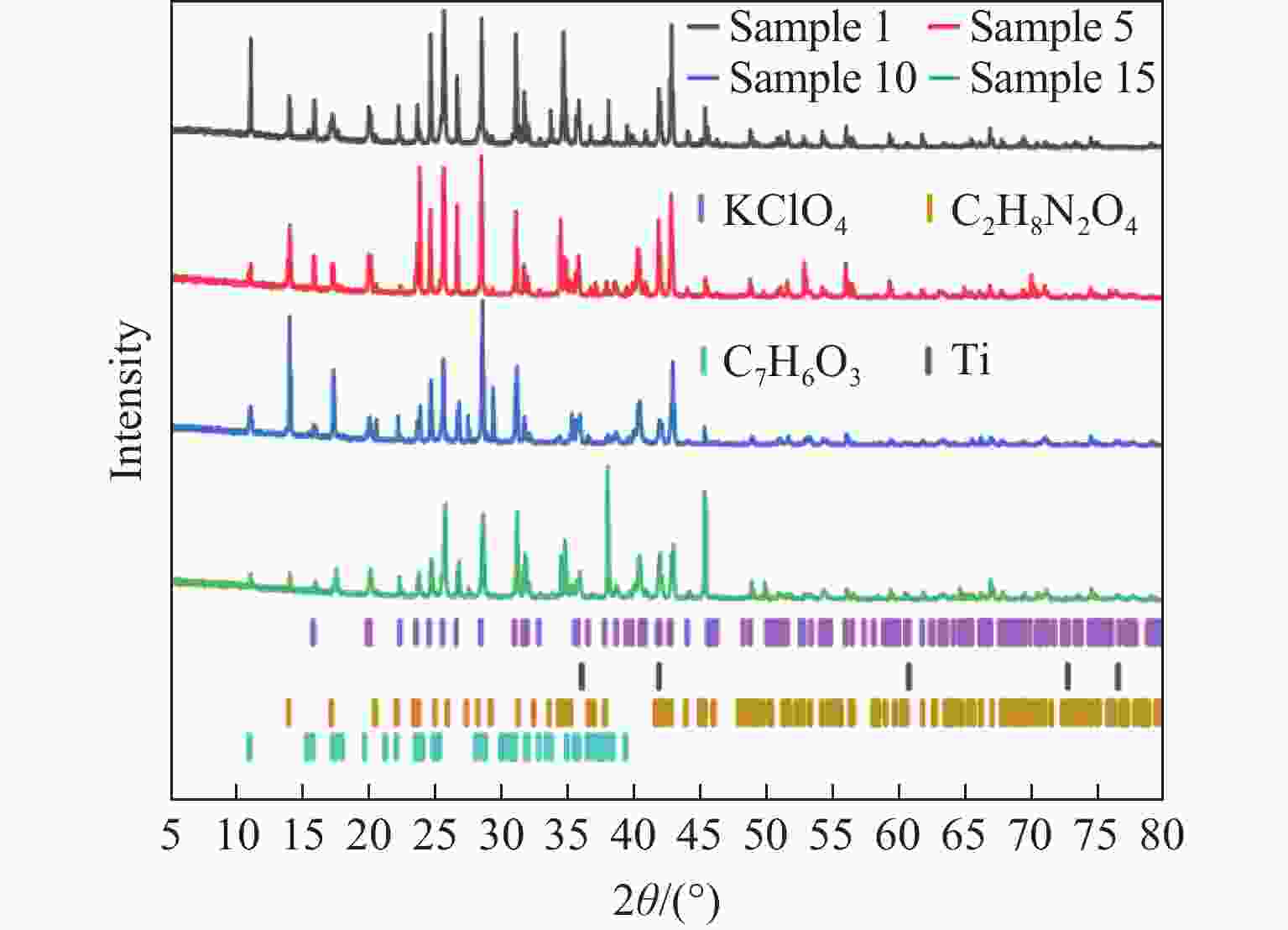
Table 7. Kinetic parameters of each sample obtained by Ozawa’ method
$ \alpha $/% E/(kJ·mol−1) r2 Sample 1 Sample 5 Sample 10 Sample 15 Sample 1 Sample 5 Sample 10 Sample 15 5 69.89 70.26 81.52 71.08 0.9933 0.9721 0.9993 0.9871 10 71.84 81.79 113.43 89.70 0.9993 0.9933 0.9993 0.9746 15 183.65 171.82 216.36 190.80 0.9715 0.9972 0.9861 0.9919 20 171.30 167.41 210.54 181.99 0.9890 0.9852 0.9942 0.9860 25 194.08 188.72 206.92 184.03 0.9815 0.9859 0.9926 0.9852 30 120.16 95.16 182.96 134.09 0.9998 0.9746 0.9509 0.9763 Average value 135.15 129.19 168.62 141.95 表 8 各样品干燥前、后的质量
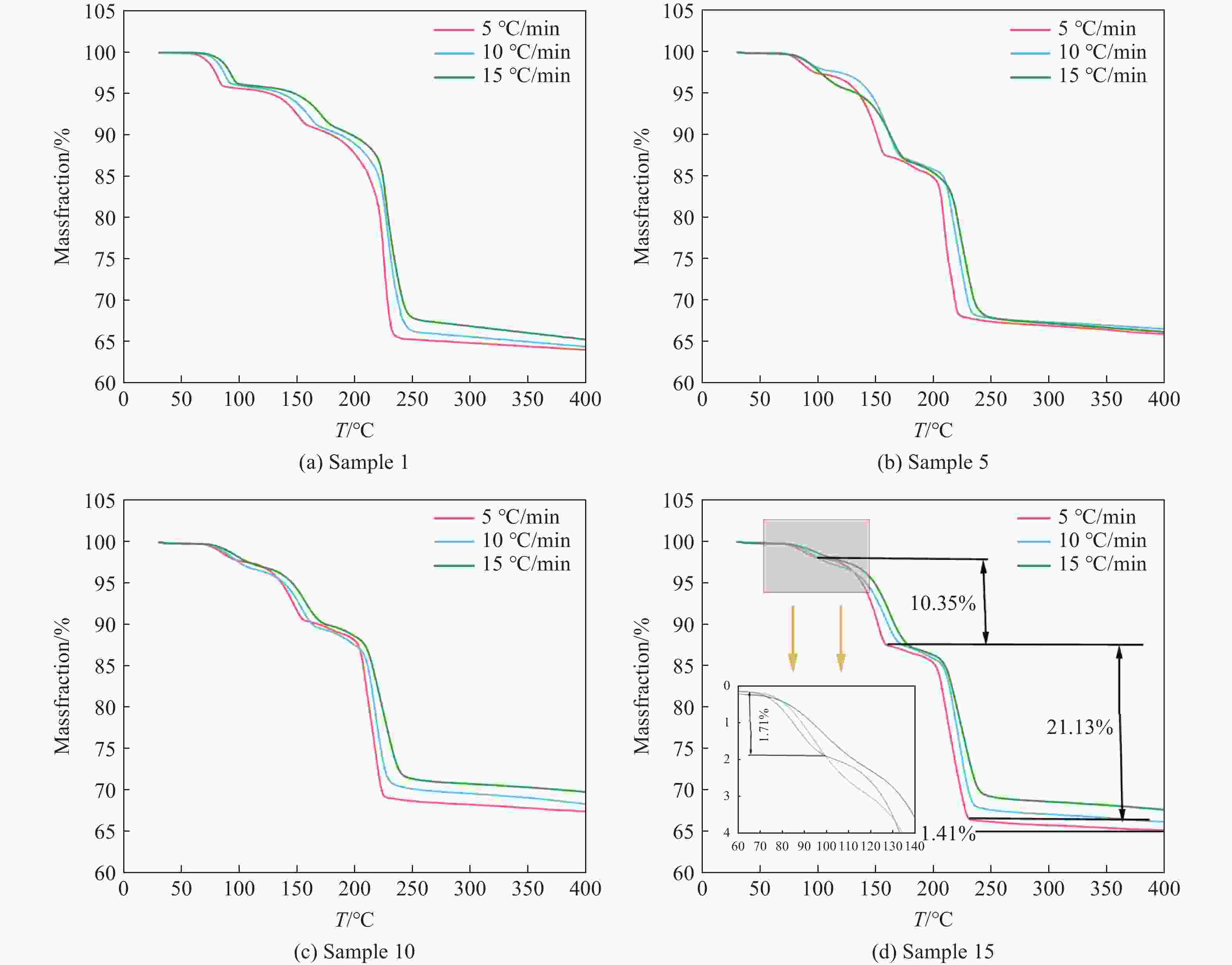
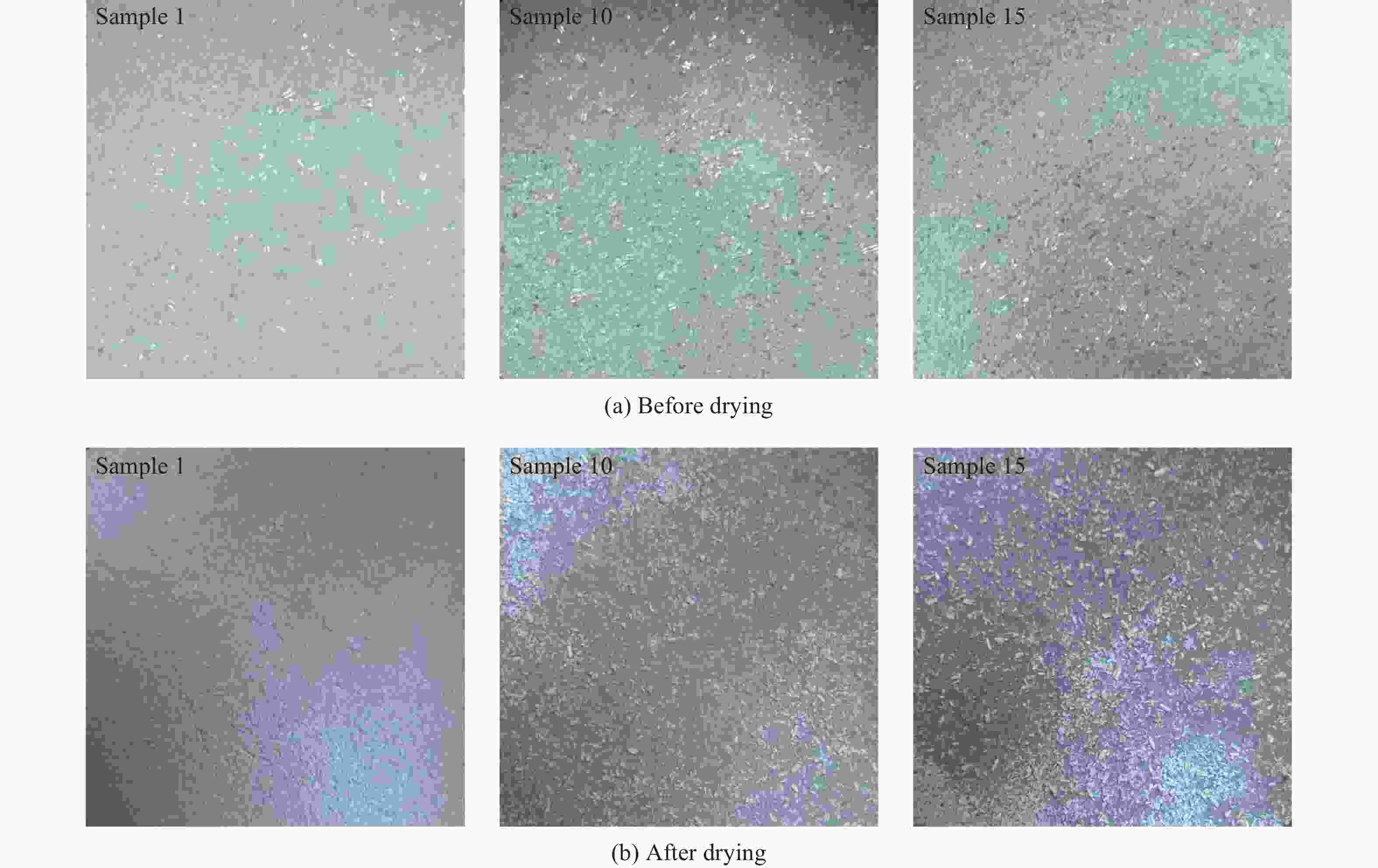
Table 8. Mass of each sample before and after drying
Sample Mass/g Hot weightlessness/% Before drying After drying 1 10.00 7.83 21.7 2 10.00 7.98 20.2 3 10.00 7.93 20.7 4 10.00 7.95 20.5 5 10.00 8.01 19.9 7 10.00 8.22 17.8 8 10.00 8.32 16.8 9 10.00 8.47 15.3 10 10.00 8.42 15.8 12 10.00 7.86 21.4 13 10.00 7.85 21.5 14 10.00 7.91 20.9 15 10.00 7.90 21.0 -
[1] 周盛涛, 罗学东, 蒋楠, 等. 二氧化碳相变致裂技术研究进展与展望 [J]. 工程科学学报, 2021, 43(7): 883–893. doi: 10.13374/j.issn2095-9389.2020.11.05.006ZHOU S T, LUO X D, JIANG N, et al. A review on fracturing technique with carbon dioxide phase transition [J]. Chinese Journal of Engineering, 2021, 43(7): 883–893. doi: 10.13374/j.issn2095-9389.2020.11.05.006 [2] 郜浩田, 付小龙, 李吉祯, 等. 硝化甘油合成及分析技术研究进展 [J]. 兵器装备工程学报, 2021, 42(11): 1–6. doi: 10.11809/bqzbgcxb2021.11.001GAO H T, FU X L, LI J Z, et al. Research progress in synthesis and analysis of nitroglycerin [J]. Journal of Ordnance Equipment Engineering, 2021, 42(11): 1–6. doi: 10.11809/bqzbgcxb2021.11.001 [3] ARMAGHANI D J, HAJIHASSANI M, SOHAEI H, et al. Neuro-fuzzy technique to predict air-overpressure induced by blasting [J]. Arabian Journal of Geosciences, 2015, 8(12): 10937–10950. doi: 10.1007/s12517-015-1984-3 [4] ZHANG Z X. Kinetic energy and its applications in mining engineering [J]. International Journal of Mining Science and Technology, 2017, 27(2): 237–244. doi: 10.1016/j.ijmst.2017.01.009 [5] 孙可明, 辛利伟, 王婷婷, 等. 超临界CO2气爆煤体致裂规律模拟研究 [J]. 中国矿业大学学报, 2017, 46(3): 50l–506. doi: 10.13247/j.cnki.jcumt.000669SUN K M, XIN L W, WANG T T, et al. Simulation research on law of coal fracture caused by supercritical CO2 explosion [J]. Journal of China University of Mining & Technology, 2017, 46(3): 50l–506. doi: 10.13247/j.cnki.jcumt.000669 [6] 孙小明. 液态二氧化碳相变致裂掏槽破岩试验研究 [J]. 煤炭科学技术, 2021, 49(8): 81–87. doi: 10.13199/j.cnki.cst.2021.08.010SUN X M. Experimental study on cutting and rock breaking by liquid CO2 phase transition fracturing technology [J]. Coal Science and Technology, 2021, 49(8): 81–87. doi: 10.13199/j.cnki.cst.2021.08.010 [7] 黄园月, 尹岚岚, 倪昊, 等. 二氧化碳致裂器研制与应用 [J]. 煤炭技术, 2015, 34(8): 123–124. doi: 10.13301/j.cnki.ct.2015.08.048HUANG Y Y, YIN L L, NI H, et al. Development and application of carbon dioxide fracturing device [J]. Coal Technology, 2015, 34(8): 123–124. doi: 10.13301/j.cnki.ct.2015.08.048 [8] 尤横, 岳中文, 杨海斌, 等. CO2相变爆破致裂管内压力变化试验研究 [J]. 工程爆破, 2023, 29(5): 105–112. doi: 10.19931/j.EB.20220365YOU H, YUE Z W, YANG H B, et al. Experimental study on pressure change in cracking tube during process of CO2 phase change blasting [J]. Engineering Blasting, 2023, 29(5): 105–112. doi: 10.19931/j.EB.20220365 [9] 张坤玉, 陈德, 吴昊. 高压气体驱动激波管的数值模拟与参数影响分析 [J]. 高压物理学报, 2023, 37(3): 033301. doi: 10.11858/gywlxb.20220704ZHANG K Y, CHEN D, WU H. Numerical simulation and parametric analysis of high-pressure gas-driven shock tube [J]. Chinese Journal of High Pressure Physics, 2023, 37(3): 033301. doi: 10.11858/gywlxb.20220704 [10] 喻健良, 郑阳光, 闫兴清, 等. 工业规模CO2管道大孔泄漏过程中的射流膨胀及扩散规律 [J]. 化工学报, 2017, 68(6): 2298–2305. doi: 10.11949/j.issn.0438-1157.20161614YU J L, ZHENG Y G, YAN X Q, et al. Under-expanded jets and dispersion during big hole leakage of high pressure CO2 pipeline in industrial scale [J]. CIESC Journal, 2017, 68(6): 2298–2305. doi: 10.11949/j.issn.0438-1157.20161614 [11] ZHANG Y N, DENG J R, DENG H W, et al. Peridynamics simulation of rock fracturing under liquid carbon dioxide blasting [J]. International Journal of Damage Mechanics, 2019, 28(7): 1038–1052. doi: 10.1177/1056789518807532 [12] 夏军, 陶良云, 李必红, 等. 二氧化碳液-气相变膨胀破岩技术及应用 [J]. 工程爆破, 2018, 24(3): 50–54. doi: 10.3969/j.issn.1006-7051.2018.03.009XIA J, TAO L Y, LI B H, et al. Technology and application of the rock breaking by CO2 liquid-gas phase transition and expansion [J]. Engineering Blasting, 2018, 24(3): 50–54. doi: 10.3969/j.issn.1006-7051.2018.03.009 [13] YU B Z, NIU S S, ZHOU S T, et al. Impact pressure characteristics of carbon dioxide phase transition fracturing technique [J]. ACS Omega, 2024, 9(22): 23927–23939. doi: 10.1021/acsomega.4c02026 [14] CAO Y X, ZHANG J S, ZHAI H, et al. CO2 gas fracturing: a novel reservoir stimulation technology in low permeability gassy coal seams [J]. Fuel, 2017, 203: 197–207. doi: 10.1016/j.fuel.2017.04.053 [15] 王长禄, 彭然, 郑义, 等. 煤层液态CO2相变致裂半径预测研究 [J]. 工矿自动化, 2023, 49(10): 110–117. doi: 10.13272/j.issn.1671-251x.2023040076WANG C L, PENG R, ZHANG Y, et al. Research on the prediction of liquid CO2 phase transition cracking radius in coal seams [J]. Journal of Mine Automation, 2023, 49(10): 110–117. doi: 10.13272/j.issn.1671-251x.2023040076 [16] 张震, 刘高峰, 李宝林, 等. CO2相变致裂煤的纳米孔隙尺度改造效应 [J]. 岩石力学与工程学报, 2023, 42(3): 672–684. doi: 10.13722/j.cnki.jrme.2022.0408ZHANG Z, LIU G F, LI B L, et al. Transformed effect of nano-pores in coal by CO2 phase transition fracturing [J]. Chinese Journal of Rock Mechanics and Engineering, 2023, 42(3): 672–684. doi: 10.13722/j.cnki.jrme.2022.0408 [17] 李豪君, 张家行, 谯永刚, 等. 液态CO2相变致裂参数及应用效果研究 [J]. 煤炭技术, 2021, 40(10): 149–152. doi: 10.13301/j.cnki.ct.2021.10.035LI H J, ZHANG J H, QIAO Y G, et al. Study on cracking parameters and application effect of liquid carbon dioxide fracturing [J]. Coal Technology, 2021, 40(10): 149–152. doi: 10.13301/j.cnki.ct.2021.10.035 [18] 宋伟. 复杂环境下地铁施工二氧化碳破岩技术研究 [J]. 铁道勘察, 2020, 46(4): 80–83, 87. doi: 10.19630/j.cnki.tdkc.201911070004SONG W. Research on carbon dioxide rock breaking technology in subway construction under complicated environment [J]. Railway Investigation and Surveying, 2020, 46(4): 80–83, 87. doi: 10.19630/j.cnki.tdkc.201911070004 [19] 李世安. 二氧化碳致裂技术在地铁车站基坑开挖中的应用 [J]. 市政技术, 2020, 38(4): 258–262, 273. doi: 10.3969/j.issn.1009-7767.2020.04.068LI S A. Application of carbon dioxide cracking technology in foundation pit excavation of metro station [J]. Municipal Engineering Technology, 2020, 38(4): 258–262, 273. doi: 10.3969/j.issn.1009-7767.2020.04.068 [20] CHANG J, SUN L J, DAI B B, et al. Research on the fracture properties and mechanism of carbon dioxide blasting based on rock-like materials [J]. Minerals, 2023, 13(1): 3. doi: 10.3390/min13010003 [21] 夏祥, 李海波, 王晓炜, 等. 核电工程中的CO2致裂与炸药爆破地表振动传播规律试验研究 [J]. 岩石力学与工程学报, 2021, 40(7): 1350–1356. doi: 10.13722/j.cnki.jrme.2020.1183XIA X, LI H B, WANG X W, et al. Comparison analysis of ground vibrations induced by CO2 gas fracturing and explosive blasting [J]. Chinese Journal of Rock Mechanics and Engineering, 2021, 40(7): 1350–1356. doi: 10.13722/j.cnki.jrme.2020.1183 [22] 孙悦, 陈先猛, 陈攀森, 等. 轻气炮低温靶的结构及液态CO2冲击压缩特性的研究 [J]. 高压物理学报, 1997, 11(2): 124–129. doi: 10.11858/gywlxb.1997.02.008SUN Y, CHEN X M, CHEN P S, et al. A kind of low temperature target system and its application in shock compression studying of liquid [J]. Chinese Journal of High Pressure Physics, 1997, 11(2): 124–129. doi: 10.11858/gywlxb.1997.02.008 [23] 陈延伟, 曹明磊, 李奇. 超临界二氧化碳相变做功发射技术研究 [J]. 兵器装备工程学报, 2024, 45(4): 224–228. doi: 10.11809/bqzbgcxb2024.04.029CHEN Y W, CAO M L, LI Q. Research progress and prospect of supercritical carbon dioxide phase transition work technology [J]. Journal of Ordnance Equipment Engineering, 2024, 45(4): 224–228. doi: 10.11809/bqzbgcxb2024.04.029 [24] 徐敏潇, 尹光春. 二氧化碳致裂器激发药剂爆炸性分类的研究 [J]. 爆破器材, 2021, 50(3): 19–22, 28. doi: 10.3969/j.issn.1001-8352.2021.03.004XU M X, YIN G C. Classification of explosive properties of simulants used in carbon dioxide splitter [J]. Explosive Materials, 2021, 50(3): 19–22, 28. doi: 10.3969/j.issn.1001-8352.2021.03.004 [25] 杨海斌, 汪旭光, 王尹军, 等. 液态CO2相变爆炸激发药剂的爆炸性与安全性 [J]. 工程爆破, 2022, 28(3): 97–102. doi: 10.19931/j.EB.20210335YANG H B, WANG X G, WANG Y J, et al. Explosiveness and safety of liquid CO2 phase change explosive excitant [J]. Engineering Blasting, 2022, 28(3): 97–102. doi: 10.19931/j.EB.20210335 [26] 杨海斌, 汪旭光, 王尹军, 等. 液态CO2相变爆炸激发药剂安全性的试验研究 [J]. 火炸药学报, 2022, 45(4): 590–596. doi: 10.14077/j.issn.1007-7812.202201011YANG H B, WANG X G, WANG Y J, et al. Experimental study on the safety of liquid CO2 phase change explosive excitant [J]. Chinese Journal of Explosives & Propellants, 2022, 45(4): 590–596. doi: 10.14077/j.issn.1007-7812.202201011 [27] 黄鲁湘, 夏军, 徐添福. 激发管药剂抗爆性能试验研究 [J]. 采矿技术, 2020, 20(6): 90–92. doi: 10.3969/j.issn.1671-2900.2020.06.024HUANG L X, XIA J, XU T F. Experimental study on the anti-explosive properties of the excitation tube agent [J]. Mining Technology, 2020, 20(6): 90–92. doi: 10.3969/j.issn.1671-2900.2020.06.024 [28] 杜明燃, 胡赏赏, 王尹军, 等. 镁粉和铝粉对CO2相变激发药剂性能的影响 [J]. 工程爆破, 2024, 30(2): 88–97. doi: 10.19931/j.EB.20220385DU M R, HU S S, WANG Y J, et al. Effect of magnesium and aluminum powders on the performance of CO2 phase change excitation agent [J]. Engineering Blasting, 2024, 30(2): 88–97. doi: 10.19931/j.EB.20220385 [29] LI X H, PEI H B, ZHANG X, et al. Cover picture: effect of aluminum particle size on the performance of aluminized explosives (Prop. , Explos. , Pyrotech. 5/2020) [J]. Propellants, Explosives, Pyrotechnics, 2020, 45(5): 687. [30] 冀威, 徐宇轩. 零氧平衡RDX/NC/AP/Al复合炸药的制备及其性能表征 [J]. 含能材料, 2022, 30(6): 528–534. doi: 10.11943/CJEM2021315JI W, XU Y X. Preparation and characterization of RDX/NC/AP/Al composite energetic microspheres based on zero-oxygen balance [J]. Chinese Journal of Energetic Materials, 2022, 30(6): 528–534. doi: 10.11943/CJEM2021315 [31] 吴翠香. 炸药爆炸产生的有毒气体对人体危害及对策 [J]. 煤矿安全, 2003, 34(6): 37–39. doi: 10.3969/j.issn.1003-496X.2003.06.015WU C X. Hazard of harmful gases caused by explosives basting on human body and countermeasures [J]. Safety in Coal Mines, 2003, 34(6): 37–39. doi: 10.3969/j.issn.1003-496X.2003.06.015 [32] 冯国富, 王晗, 汪长栓, 等. 桥塞投放工具用低残渣低燃速复合推进剂 [J]. 火炸药学报, 2008, 31(2): 71–74. doi: 10.3969/j.issn.1007-7812.2008.02.019FENG G F, WANG H, WANG C S, et al. Composite propellant with low residue percentage and low burning rate used in the bridge-plug equipment [J]. Chinese Journal of Explosives & Propellants, 2008, 31(2): 71–74. doi: 10.3969/j.issn.1007-7812.2008.02.019 [33] 王彩玲, 赵省向, 贾铭, 等. 含AP非理想炸药爆轰产物分析与计算 [J]. 含能材料, 2014, 22(2): 235–239. doi: 10.3969/j.issn.1006-9941.2014.02.022WANG C L, ZHAO S X, JIA M, et al. Calculation of detonation products for non-ideal explosive with AP [J]. Chinese Journal of Energetic Materials, 2014, 22(2): 235–239. doi: 10.3969/j.issn.1006-9941.2014.02.022 [34] 李世伟, 王正宏, 吴成成, 等. 铝粉含量对RDX基含铝炸药爆热性能的影响 [J]. 爆破器材, 2022, 51(4): 29–32. doi: 10.3969/j.issn.1001-8352.2022.04.005LI S W, WANG Z H, WU C C, et al. Effect of aluminum content on detonation heat of RDX-based aluminized explosives [J]. Explosive Materials, 2022, 51(4): 29–32. doi: 10.3969/j.issn.1001-8352.2022.04.005 [35] 王冬梅, 耿志远. 草酸热分解机理的DFT研究 [J]. 化学与生物工程, 2011, 28(3): 39–43. doi: 10.3969/j.issn.1672-5425.2011.03.011WANG D M, GENG Z Y. DFT study on thermal decomposition mechanism of oxalic acid [J]. Chemistry & Bioengineering, 2011, 28(3): 39–43. doi: 10.3969/j.issn.1672-5425.2011.03.011 [36] CUI H W, JIU J T, NAGAO S, et al. Using Ozawa method to study the curing kinetics of electrically conductive adhesives [J]. Journal of Thermal Analysis and Calorimetry, 2014, 117(3): 1365–1373. doi: 10.1007/s10973-014-3902-4 [37] 孙亚伦, 刘璐, 任慧, 等. 锆粉对高氯酸钾热分解反应的影响 [J]. 含能材料, 2017, 25(5): 396–402. doi: 10.11943/j.issn.1006-9941.2017.05.008SUN Y L, LIU L, REN H, et al. Effect of zirconium powder on thermal decomposition of KClO4 [J]. Chinese Journal of Energetic Materials, 2017, 25(5): 396–402. doi: 10.11943/j.issn.1006-9941.2017.05.008 [38] 王国强, 李勇宏, 胥会祥, 等. 高氯酸钾复合推进剂的耐温性 [J]. 火炸药学报, 2015, 38(5): 83–86. doi: 10.14077/j.issn.1007-7812.2015.05.017WANG G Q, LI Y H, XU H X, et al. Temperature resistance of potassium perchlorate composite propellant [J]. Chinese Journal of Explosives & Propellants, 2015, 38(5): 83–86. doi: 10.14077/j.issn.1007-7812.2015.05.017 [39] 朱铭铮. 基于DMA技术的环氧树脂耐热性能研究 [D]. 武汉: 武汉理工大学, 2005.ZHU M Z. Study on thermal properties of epoxy resins by DMA [D]. Wuhan: Wuhan University of Technology, 2005. [40] 郑剑, 陈人杰, 李国平, 等. RDX/AP分子间炸药热性能的研究 [J]. 北京理工大学学报, 2011, 31(4): 482–485. doi: 10.15918/j.tbit1001-0645.2011.04.007ZHENG J, CHEN R J, LI G P, et al. Study on thermal decomposition of RDX/AP intermolecular explosive [J]. Transactions of Beijing Institute of Technology, 2011, 31(4): 482–485. doi: 10.15918/j.tbit1001-0645.2011.04.007 -







 下载:
下载: