Crystal Structure and Physica Properties of Perovskite Oxide BaMO3 (M Being Transition Metal)
-
摘要: 钙钛矿氧化物BaMO3(M为过渡族金属)具有复杂的晶体结构和物理性质,本文系统地总结了BaMO3的研究进展,重点关注在 M 元素变化过程中晶体结构和物理性质的演化,以及高压调控下的结构相变、电输运性质和磁学性质的变化,讨论了M离子半径及合成压力对六方钙钛矿到钙钛矿演化过程的影响,同时对该领域中一些问题做了展望,探讨了这一体系可能出现的新的原子组合和结构,相应材料可能具有的新特性和科学意义。Abstract: The perovskite oxide BaMO3 (M being transition metal) has a complex crystal structure and physical properties. This article systematically summarizes the research progress, focusing on the evolution of crystal structure and physical properties during the M element change process, as well as the structural phase transition, electrical transport properties, and magnetic properties regulation under high-pressure. The influence of M ion radius and synthesis pressure on the evolution process from hexagonal perovskite to perovskite is discussed, and some issues in this field are also discussed. The possible new atomic combinations and structures in this system, as well as the new characteristics and scientific significance of these corresponding materials, are discussed.
-
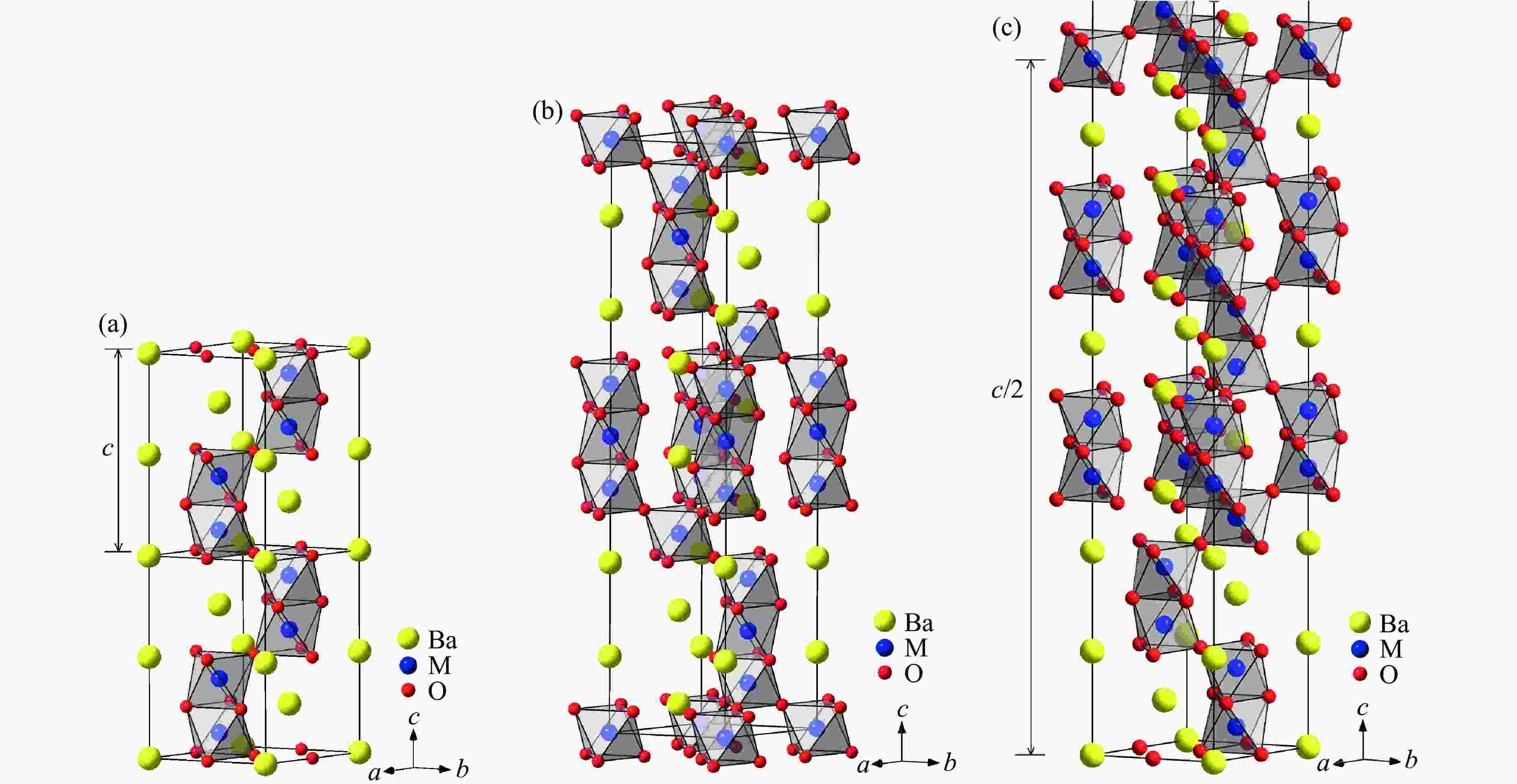
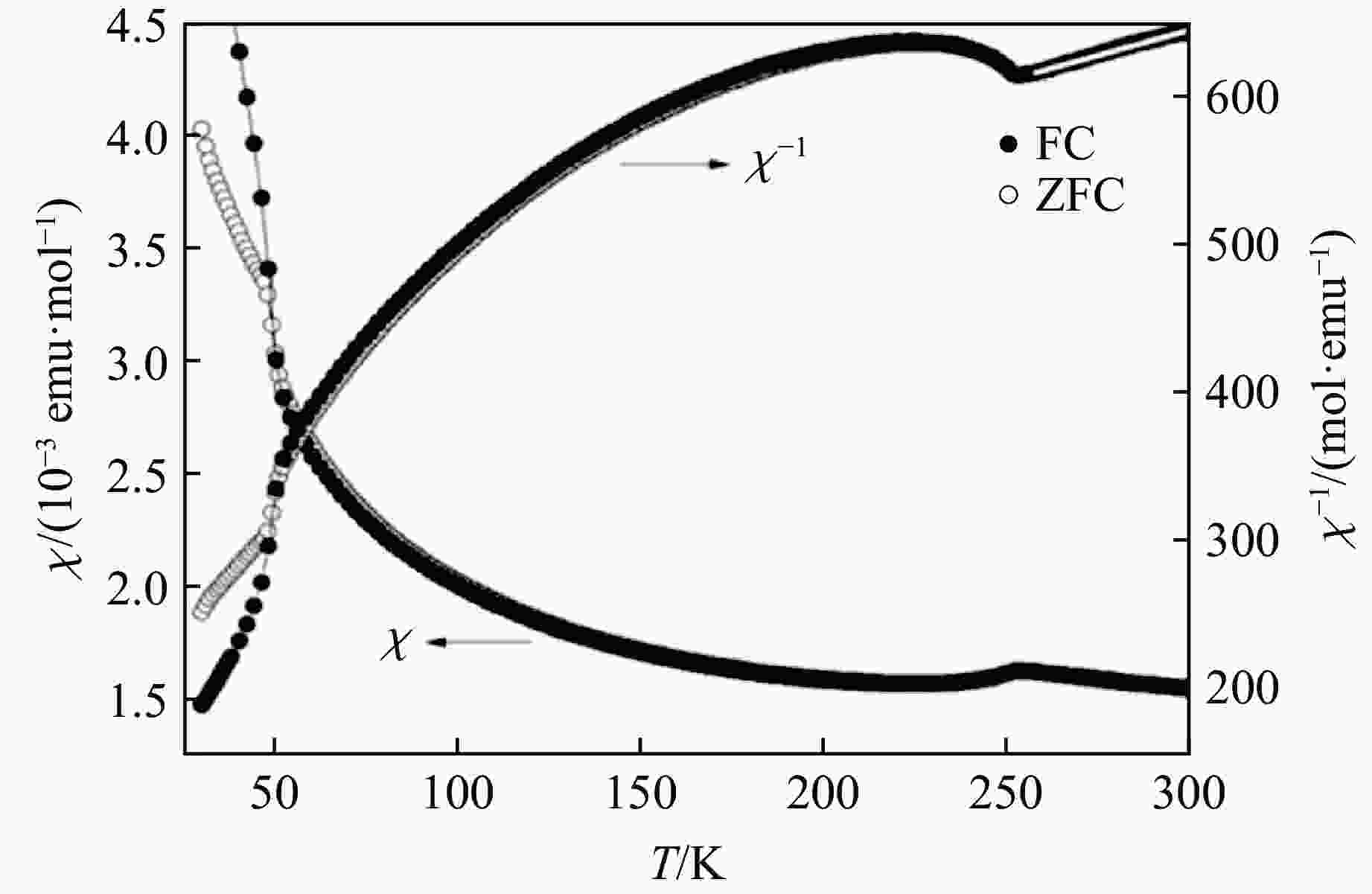
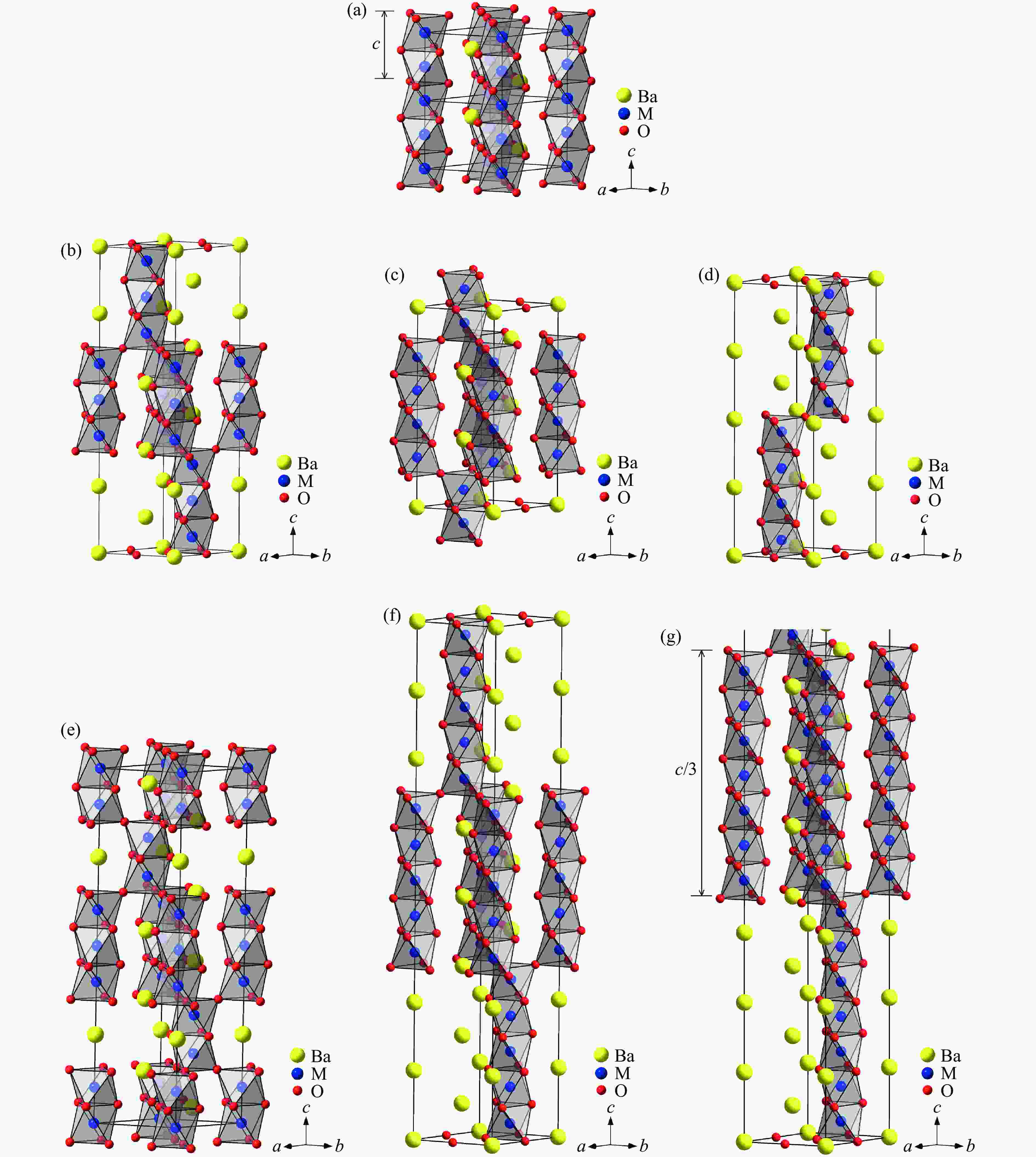
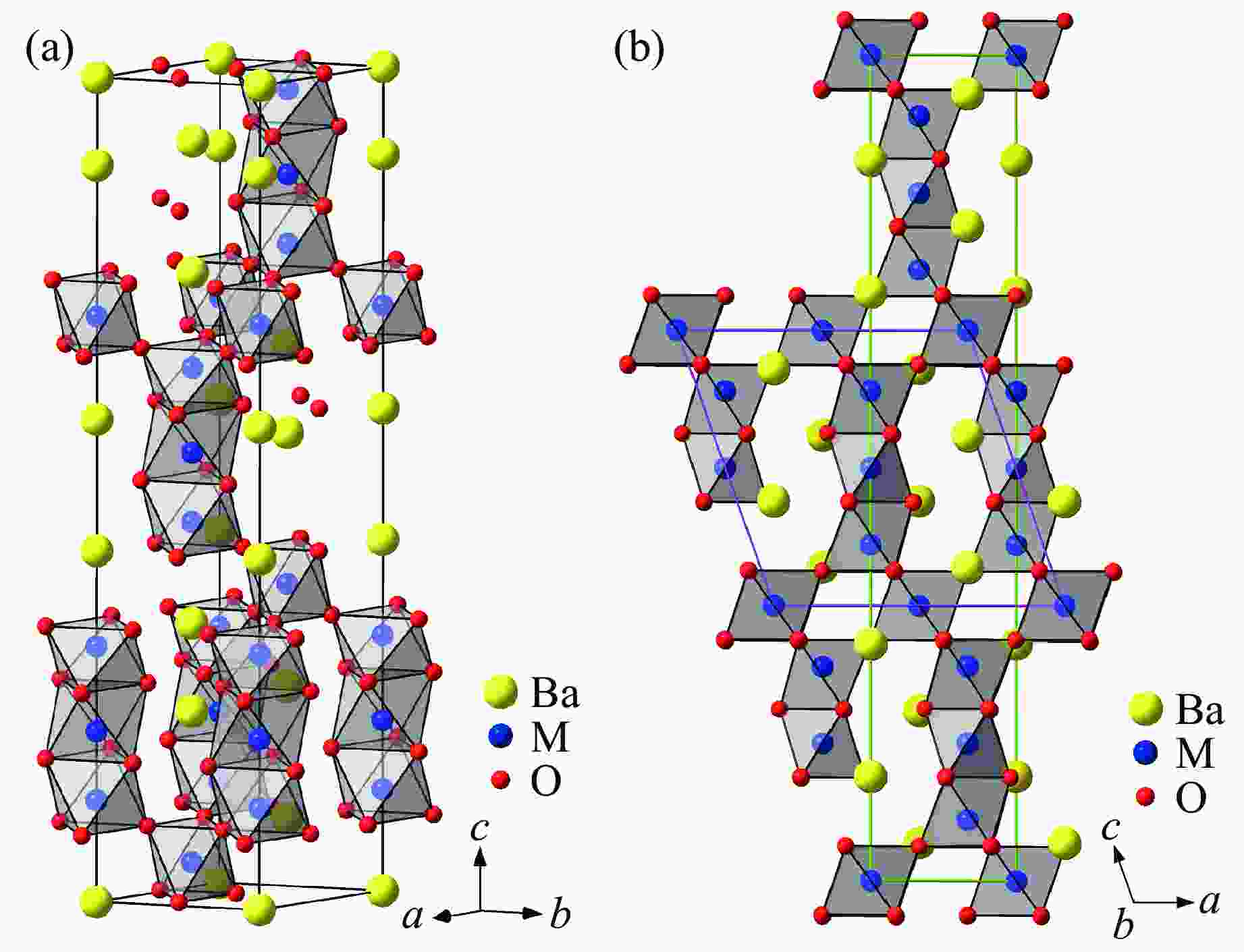
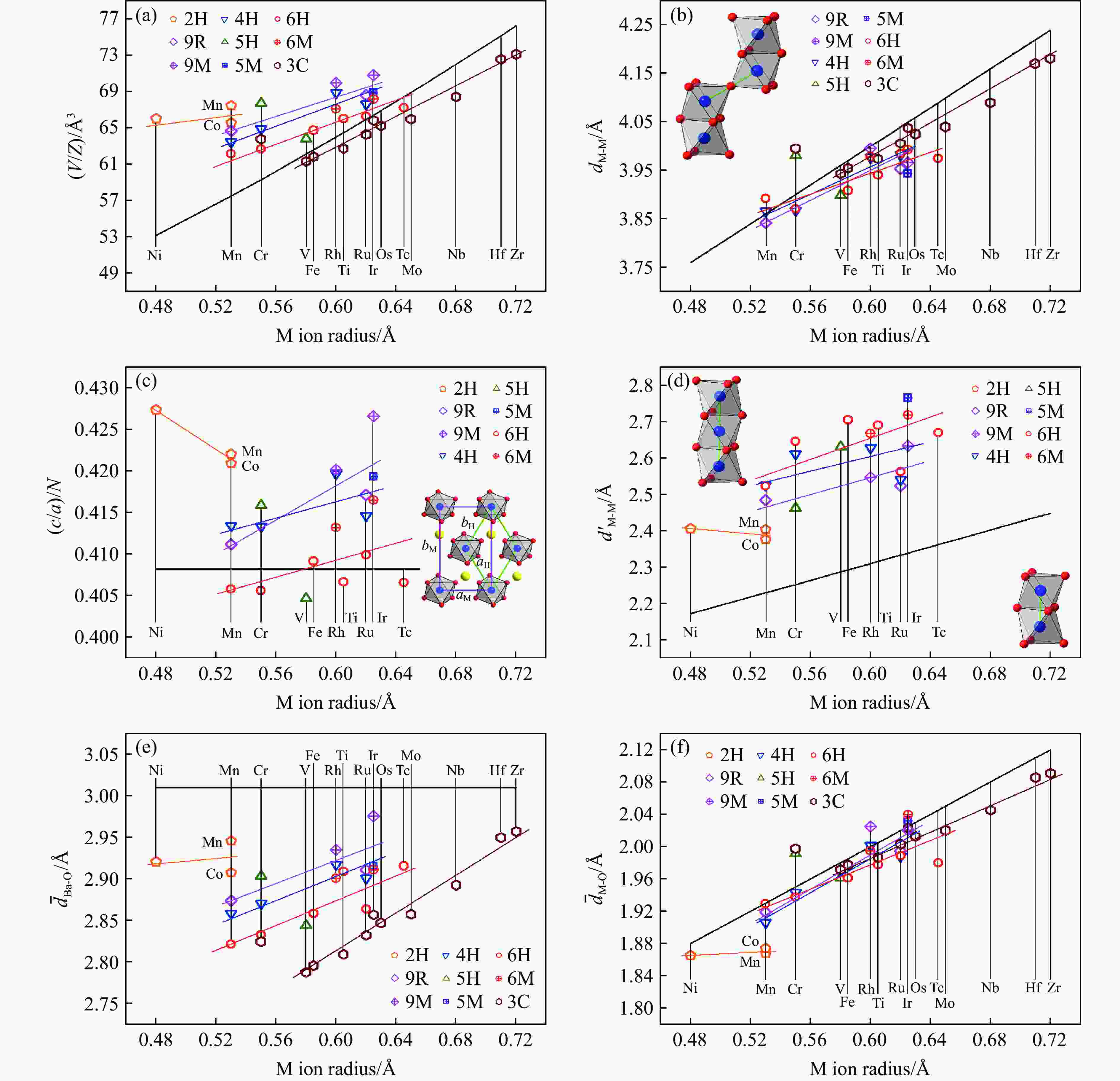
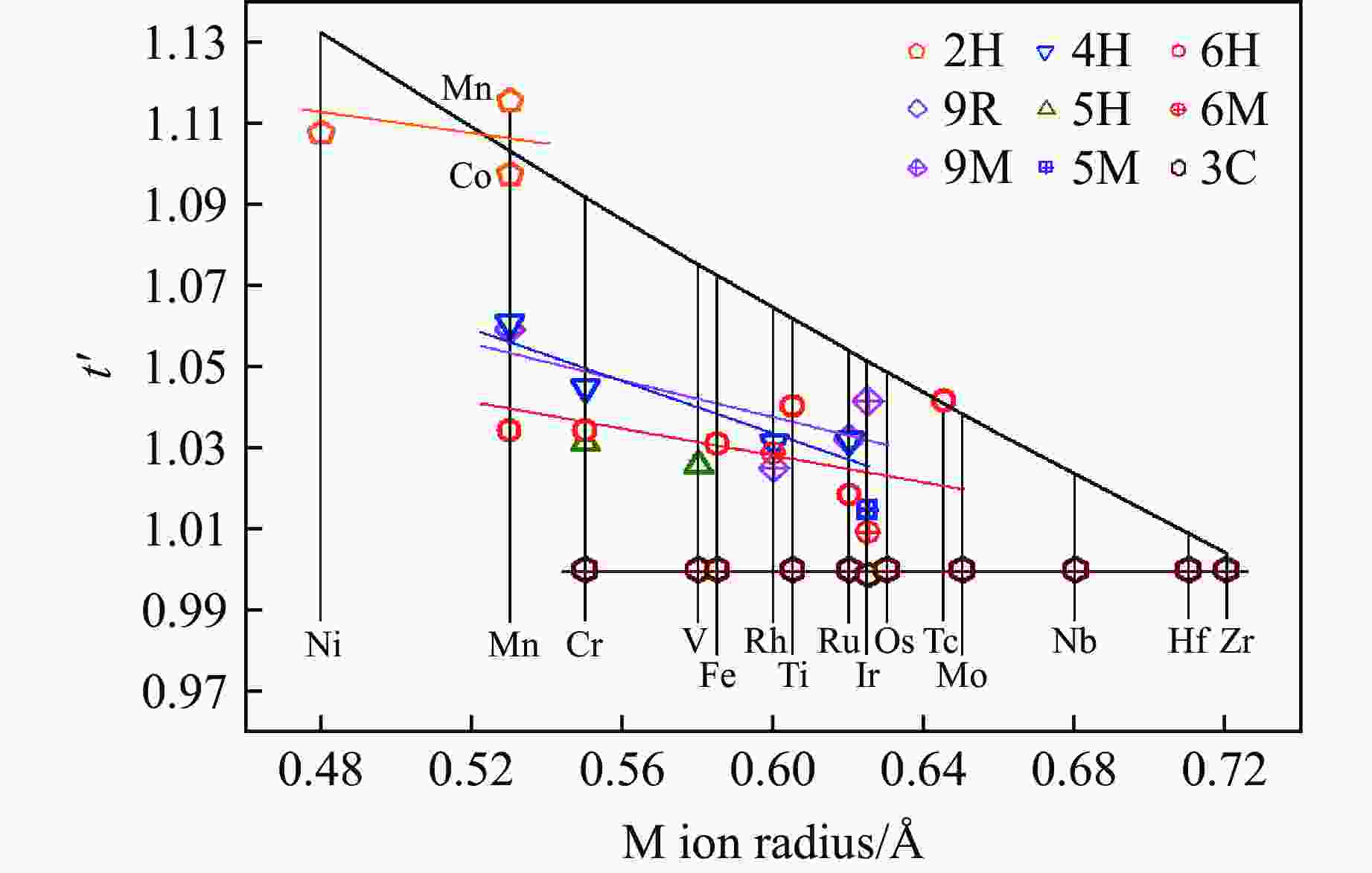
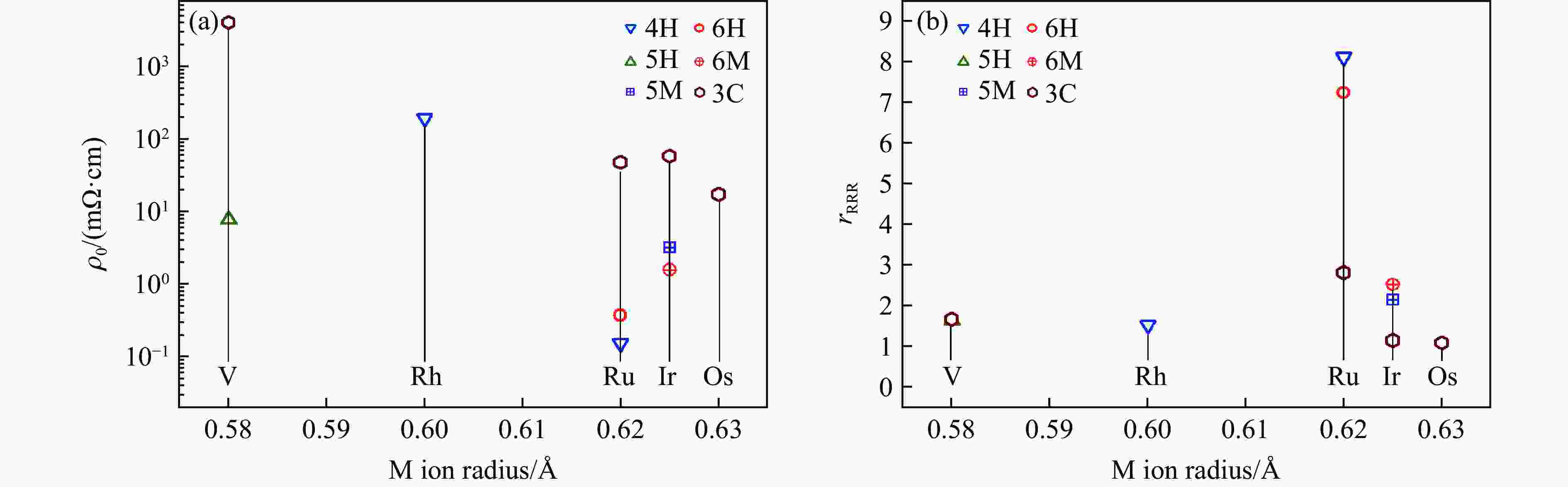
图 29 BaMO3的(a) 每个化学式的晶胞体积V/Z、(b) 近邻多聚体之间的M-M距离dM-M、(c) 轴比率(c/a)/N、(d) 多聚体内M-M之间的距离
${d'_{{\text{M-M}}}} $ ,(e) Ba-O之间的平均距离${\bar d_{{\text{Ba-O}}}}$ 和(f) M-O之间的平均距离${\bar d_{{\text{M-O}}}}$ 随M离子半径的变化关系Figure 29. Relations of (a) volume per chemical formula V/Z, (b) M-M distance between neighbour polymers dM-M,(c) axis ratio (c/a)/N, (d) M-M distance in one polymer
${d'_{{\text{M-M}}}} $ ; (e) average distance of Ba-O${\bar d_{{\text{Ba-O}}}}$ ; (f) average distance of M-O${\bar d_{{\text{M-O}}}}$ versus M ion radius of BaMO3 -
[1] GOLDSCHMIDT V M. Die gesetze der krystallochemie [J]. Naturwissenschaften, 1926, 14(21): 477–485. doi: 10.1007/BF01507527 [2] GLAZER A M. The classification of tilted octahedra in perovskites [J]. Acta Crystallographica Section B, 1972, 28(11): 3384–3392. doi: 10.1107/S0567740872007976 [3] SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides [J]. Acta Crystallographica Section A, 1976, 32(5): 751–767. doi: 10.1107/S0567739476001551 [4] NGUYEN L T, CAVA R J. Hexagonal perovskites as quantum materials [J]. Chemical Reviews, 2021, 121(5): 2935–2965. doi: 10.1021/acs.chemrev.0c00622 [5] AKIMOTO J, GOTOH Y, OSAWA Y. Refinement of hexagonal BaTiO3 [J]. Acta Crystallographica Section C, 1994, 50(2): 160–161. [6] HAYWARD S A, REDFERN S A T, STONE H J, et al. Phase transitions in BaTiO3: a high-pressure neutron diffraction study [J]. Zeitschrift für Kristallographie-Crystalline Materials, 2005, 220(8): 735–739. [7] LIU G, GREEDAN J E. Syntheses, structures, and characterization of 5-layer BaVO3− x (x = 0.2, 0.1, 0.0) [J]. Journal of Solid State Chemistry, 1994, 110(2): 274–289. doi: 10.1006/jssc.1994.1170 [8] NISHIMURA K, YAMADA I, OKA K, et al. High-pressure synthesis of BaVO3: a new cubic perovskite [J]. Journal of Physics and Chemistry of Solids, 2014, 75(6): 710–712. doi: 10.1016/j.jpcs.2014.02.001 [9] CHAMBERLAND B L, DANIELSON P S. Alkaline-earth vanadium (Ⅳ) oxides having the AVO3 composition [J]. Journal of Solid State Chemistry, 1971, 3(2): 243–247. doi: 10.1016/0022-4596(71)90035-1 [10] ARÉVALO-LÓPEZ A M, ATTFIELD J P. High-pressure BaCrO3 polytypes and the 5H-BaCrO2.8 phase [J]. Journal of Solid State Chemistry, 2015, 232: 236–240. doi: 10.1016/j.jssc.2015.09.029 [11] CHAMBERLAND B L. Crystal structure of the 4H BaCrO3 polytype [J]. Journal of Solid State Chemistry, 1982, 43(3): 309–313. doi: 10.1016/0022-4596(82)90245-6 [12] CHAMBERLAND B L. Crystal structure of the 6H BaCrO3 polytype [J]. Journal of Solid State Chemistry, 1983, 48(3): 318–322. doi: 10.1016/0022-4596(83)90088-9 [13] CHAMBERLAND B L. Preparation and crystallographic properties of barium chromate (Ⅳ) polytypes [J]. Inorganic Chemistry, 1969, 8(2): 286–290. doi: 10.1021/ic50072a021 [14] CHAMBERLAND B L, KATZ L. The structure of the fourteen-layer polytype of barium chromium trioxide, BaCrO3 [J]. Acta Crystallographica Section B, 1982, 38(1): 54–57. doi: 10.1107/S0567740882002039 [15] HARADEM P S, CHAMBERLAND B L, KATZ L. The structure of the 27-layer polytype of BaCrO3 [J]. Journal of Solid State Chemistry, 1980, 34(1): 59–64. doi: 10.1016/0022-4596(80)90403-X [16] ARÉVALO-LÓPEZ A M, REEVES S J, ATTFIELD J P. Ferrimagnetism in the high pressure 6H-perovskite BaCrO3 [J]. Zeitschrift für Anorganische und Allgemeine Chemie, 2014, 640(14): 2727–2729. [17] CUSSEN E J, BATTLE P D. Crystal and magnetic structures of 2H BaMnO3 [J]. Chemistry of Materials, 2000, 12(3): 831–838. doi: 10.1021/cm991144j [18] SYONO Y, AKIMOTO S I, KOHN K. Structure relations of hexagonal perovskite-like compounds ABX3 at high pressure [J]. Journal of the Physical Society of Japan, 1969, 26(4): 993–999. doi: 10.1143/JPSJ.26.993 [19] CHRISTENSEN A N, OLLIVIER G. Hydrothermal and high-pressure preparation of some BaMnO3 modifications and low-temperature magnetic properties of BaMnO3(2H) [J]. Journal of Solid State Chemistry, 1972, 4(1): 131–137. doi: 10.1016/0022-4596(72)90141-7 [20] BOULLAY P, HERVIEU M, LABBÉ P, et al. Single crystal and HREM study of the “Bi-Sr” stabilized BaMnO3 9R polytype [J]. Materials Research Bulletin, 1997, 32(1): 35–42. doi: 10.1016/S0025-5408(96)00169-9 [21] HARDY A. Structures cristallines de deux variétés allotropiques de manganite de baryum. Nouvelle structure ABO3 [J]. Acta Crystallographica, 1962, 15(3): 179–181. doi: 10.1107/S0365110X6200047X [22] QIN S J, CHIN Y Y, ZHOU B W, et al. High-pressure synthesis and magnetism of the 4H-BaMnO3 single crystal and its 6H-type polymorph [J]. Inorganic Chemistry, 2021, 60(21): 16308–16315. doi: 10.1021/acs.inorgchem.1c02155 [23] ADKIN J J, HAYWARD M A. BaMnO3− x revisited: a structural and magnetic study [J]. Chemistry of Materials, 2007, 19(4): 755–762. doi: 10.1021/cm062055r [24] POTOFF A D, CHAMBERLAND B L, KATZ L. A single crystal study of eight-layer barium managanese oxide, BaMnO3 [J]. Journal of Solid State Chemistry, 1973, 8(3): 234–237. doi: 10.1016/0022-4596(73)90090-X [25] PARRAS M, GONZÁLEZ-CALBET J M, ALONSO J, et al. Microstructural characterization of BaMnO3− y (0.08 ≤ y ≤ 0.12): evidence for a new polytype (21R) [J]. Journal of Solid State Chemistry, 1994, 113(1): 78–87. doi: 10.1006/jssc.1994.1344 [26] POOJITHA B, RATHORE A, KUMAR A, et al. Signatures of magnetostriction and spin-phonon coupling in magnetoelectric hexagonal 15R-BaMnO3 [J]. Physical Review B, 2020, 102(13): 134436. doi: 10.1103/PhysRevB.102.134436 [27] KORNETA O B, QI T F, GE M, et al. Correlated giant dielectric peaks and antiferromagnetic transitions near room temperature in pure and alkali-doped BaMnO3-δ [J]. Journal of Physics: Condensed Matter, 2011, 23(43): 435901. doi: 10.1088/0953-8984/23/43/435901 [28] GONZÁLEZ-CALBET J M, PARRAS M, ALONSO J, et al. Prediction of novel BaMnO3− y (0 < y < 0.1) perovskite related phases [J]. Journal of Solid State Chemistry, 1994, 111(1): 202–207. doi: 10.1006/jssc.1994.1218 [29] PARRAS M, VALLET-REGI M, GONZALEZ-CALBET J M, et al. A reassessment of Ba2Fe2O5 [J]. Materials Research Bulletin, 1987, 22(10): 1413–1419. doi: 10.1016/0025-5408(87)90306-0 [30] MORI K, KAMIYAMA T, KOBAYASHI H, et al. Structural evidence for the charge disproportionation of Fe4+ in BaFeO3−δ [J]. Journal of the Physical Society of Japan, 2003, 72(8): 2024–2028. doi: 10.1143/JPSJ.72.2024 [31] MORI K, KAMIYAMA T, KOBAYASHI H, et al. Mixed magnetic phase in 6H-type BaFeO3− δ [J]. Journal of Applied Crystallography, 2007, 40(Suppl 1): s501–s505. doi: 10.1107/S0021889807001653 [32] GÓMEZ M I, LUCOTTI G, DE MORÁN J A, et al. Ab initio structure solution of BaFeO2.8− δ, a new polytype in the system BaFeO y (2.5 ≤ y ≤ 3.0) prepared from the oxidative thermal decomposition of BaFe[(CN)5NO]·3H2O [J]. Journal of Solid State Chemistry, 2001, 160(1): 17–24. doi: 10.1006/jssc.2001.9119 [33] PARRAS M, VALLETREGI M, GONZALEZCALBET J M, et al. A structural study of 12H-BaFeO2.93 [J]. European Journal of Solid State and Inorganic Chemistry, 1989, 26(3): 299–312. [34] TAN Z H, ROMERO F D, SAITO T, et al. Charge disproportionation and interchange transitions in twelve-layer BaFeO3 [J]. Physical Review B, 2020, 102(5): 054404. doi: 10.1103/PhysRevB.102.054404 [35] HAYASHI N, YAMAMOTO T, KAGEYAMA H, et al. BaFeO3: a ferromagnetic iron oxide [J]. Angewandte Chemie International Edition, 2011, 50(52): 12547–12550. [36] MIZUMAKI M, YOSHII K, HAYASHI N, et al. Magnetocaloric effect of field-induced ferromagnet BaFeO3 [J]. Journal of Applied Physics, 2013, 114(7): 073901. doi: 10.1063/1.4818316 [37] LIU Y X, LIU Z H, LI Z, et al. Multiple magnetic transitions and electrical transport transformation of a BaFeO3 cubic perovskite single crystal [J]. Physical Review B, 2020, 101(14): 144421. doi: 10.1103/PhysRevB.101.144421 [38] STRAUSS S W, FANKUCHEN I, WARD R. Barium cobalt oxide of the perowskite type [J]. Journal of the American Chemical Society, 1951, 73(11): 5084–5086. doi: 10.1021/ja01155a019 [39] TAGUCHI H, TAKEDA Y, KANAMARU F, et al. Cobalt trioxide [J]. Acta Crystallographica Section B, 1977, 33(4): 1298–1299. doi: 10.1107/S0567740877005937 [40] WANG H D, YANG J H, DONG C H, et al. Crystal growth and characterization of the quasi-one-dimensional compound BaCoO3 [J]. Journal of Crystal Growth, 2015, 430: 52–54. doi: 10.1016/j.jcrysgro.2015.08.010 [41] SUGIYAMA J, NOZAKI H, BREWER J H, et al. Appearance of a two-dimensional antiferromagnetic order in quasi-one-dimensional cobalt oxides [J]. Physical Review B, 2005, 72(6): 064418. doi: 10.1103/PhysRevB.72.064418 [42] NOZAKI H, JANOSCHEK M, ROESSLI B, et al. Neutron diffraction and μSR study on the antiferromagnet BaCoO3 [J]. Physical Review B, 2007, 76(1): 014402. doi: 10.1103/PhysRevB.76.014402 [43] BOTTA P M, PARDO V, BALDOMIR D, et al. Dynamic magnetic behavior of BaCoO3 quasi-one-dimensional perovskite [J]. Physical Review B, 2006, 74(21): 214415. doi: 10.1103/PhysRevB.74.214415 [44] WANG H Z, XU X H, NI D R, et al. Impersonating a superconductor: high-pressure BaCoO3, an insulating ferromagnet [J]. Journal of the American Chemical Society, 2023, 145(39): 21203-21206. [45] JACOBSON A J, HUTCHISON J L. An investigation of the structure of 12H BaCoO2.6 by electron microscopy and powder neutron diffraction [J]. Journal of Solid State Chemistry, 1980, 35(3): 334–340. doi: 10.1016/0022-4596(80)90530-7 [46] PARRAS M, VARELA A, SEEHOFER H, et al. HREM study of the BaCoO3− y system: evidence for a new 5H phase [J]. Journal of Solid State Chemistry, 1995, 120(2): 327–331. doi: 10.1006/jssc.1995.1416 [47] MENTRÉ O, IORGULESCU M, HUVÉ M, et al. BaCoO2.22: the most oxygen-deficient certified cubic perovskite [J]. Dalton Transactions, 2015, 44(23): 10728–10737. [48] LANDER J J. The crystal structures of NiO·BaO3, NiO·BaO, BaNiO3 and intermediate phases with composition near Ba2Ni2O5, with a note on NiO [J]. Acta Crystallographica, 1951, 4(2): 148–156. doi: 10.1107/S0365110X51000441 [49] TAKEDA Y, SHIMADA M, KANAMARU F, et al. Preparation and magnetic property of BaNiO3 single crystals [J]. Chemistry Letters, 1974, 3(2): 107–108. doi: 10.1246/cl.1974.107 [50] DONOHUE P C, KATZ L, WARD R. The crystal structure of barium ruthenium oxide and related compounds [J]. Inorganic Chemistry, 1965, 4(3): 306–310. doi: 10.1021/ic50025a010 [51] RAO M V R, SATHE V G, SORNADURAI D, et al. Electronic structure of ARuO3 (A = Ca, Sr and Ba) compounds [J]. Journal of Physics and Chemistry of Solids, 2001, 62(4): 797–806. doi: 10.1016/S0022-3697(00)00262-6 [52] HONG S T, SLEIGHT A W. Crystal structure of 4H BaRuO3: high pressure phase prepared at ambient pressure [J]. Journal of Solid State Chemistry, 1997, 128(2): 251–255. [53] RIJSSENBEEK J T, JIN R, ZADOROZHNY Y, et al. Electrical and magnetic properties of the two crystallographic forms of BaRuO3 [J]. Physical Review B, 1999, 59(7): 4561–4564. doi: 10.1103/PhysRevB.59.4561 [54] ZHAO J G, YANG L X, YU Y, et al. Structural and physical properties of the 6H BaRuO3 polymorph synthesized under high pressure [J]. Journal of Solid State Chemistry, 2007, 180(10): 2816–2823. doi: 10.1016/j.jssc.2007.07.031 [55] JIN C Q, ZHOU J S, GOODENOUGH J B, et al. High-pressure synthesis of the cubic perovskite BaRuO3 and evolution of ferromagnetism in ARuO3 (A = Ca, Sr, Ba) ruthenates [J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(20): 7115–7119. [56] OGAWA T, SATO H. New ternary barium ruthenates: 10H-type BaRuO3 and Ba2Ru7O18 [J]. Journal of Alloys and Compounds, 2004, 383(1/2): 313–318. [57] ZHOU J S, MATSUBAYASHI K, UWATOKO Y, et al. Critical behavior of the ferromagnetic perovskite BaRuO3 [J]. Physical Review Letters, 2008, 101(7): 077206. doi: 10.1103/PhysRevLett.101.077206 [58] NEUMEIER J J, CORNELIUS A L, SCHILLING J S. Influence of pressure on the ferromagnetic transition temperature of SrRuO3 [J]. Physica B: Condensed Matter, 1994, 198(4): 324–328. [59] SIEGRIST T, CHAMBERLAND B L. The crystal structure of BaIrO3 [J]. Journal of the Less Common Metals, 1991, 170(1): 93–99. doi: 10.1016/0022-5088(91)90054-8 [60] CHENG J G, ALONSO J A, SUARD E, et al. A new perovskite polytype in the high-pressure sequence of BaIrO3 [J]. Journal of the American Chemical Society, 2009, 131(21): 7461–7469. [61] ZHAO J G, YANG L X, YU Y, et al. Physical properties of the 5M BaIrO3: a new weak ferromagnetic iridate synthesized under high pressure [J]. Solid State Communications, 2010, 150(1): 36–39. [62] ZHAO J G, YANG L X, YU Y, et al. Structural and physical properties of the 6M BaIrO3: a new metallic iridate synthesized under high pressure [J]. Inorganic Chemistry, 2009, 48(10): 4290–4294. [63] CHENG J G, ISHII T, KOJITANI H, et al. High-pressure synthesis of the BaIrO3 perovskite: a Pauli paramagnetic metal with a Fermi liquid ground state [J]. Physical Review B, 2013, 88(20): 205114. doi: 10.1103/PhysRevB.88.205114 [64] CHENG J G, ZHOU J S, ALONSO J A, et al. Transition from a weak ferromagnetic insulator to an exchange-enhanced paramagnetic metal in the BaIrO3 polytypes [J]. Physical Review B, 2009, 80(10): 104430. doi: 10.1103/PhysRevB.80.104430 [65] CAO G, CROW J E, GUERTIN R P, et al. Charge density wave formation accompanying ferromagnetic ordering in quasi-one-dimensional BaIrO3 [J]. Solid State Communications, 2000, 113(11): 657–662. doi: 10.1016/S0038-1098(99)00532-3 [66] POWELL A V, BATTLE P D. The electronic properties of non-stoichiometric barium iridate, BaIrO3− δ [J]. Journal of Alloys and Compounds, 1993, 191(2): 313–318. doi: 10.1016/0925-8388(93)90085-2 [67] ZHAO J G, YANG L X, MYDEEN K, et al. Effects of pressure on electrical property of BaIrO3 [J]. Solid State Communications, 2008, 148(9/10): 361–364. [68] KIDA T, SENDA A, YOSHII S, et al. Pressure effect on magnetic properties of a weak ferromagnet BaIrO3 [J]. Journal of Physics: Conference Series, 2010, 200(1): 012084. doi: 10.1088/1742-6596/200/1/012084 [69] SIEGRIST T, LARSON E M, CHAMBERLAND B L. Structural studies of high-pressure Ba-Rh-O phases [J]. Journal of Alloys and Compounds, 1994, 210(1/2): 13–17. [70] CHAMBERLAND B L, ANDERSON J B. The preparation and crystal structure of a BaRhO3 polytype [J]. Journal of Solid State Chemistry, 1981, 39(1): 114–119. doi: 10.1016/0022-4596(81)90309-1 [71] INJAC S D A, XU Y H, ROMERO F D, et al. Pauli-paramagnetic and metallic properties of high pressure polymorphs of BaRhO3 oxides containing Rh2O9 dimers [J]. Dalton Transactions, 2021, 50(13): 4673–4679. doi: 10.1039/D1DT00502B [72] MEGAW H D. Crystal structure of double oxides of the perovskite type [J]. Proceedings of the Physical Society, 1946, 58(2): 133–152. doi: 10.1088/0959-5309/58/2/301 [73] KOPNIN E M, ISTOMIN S Y, D’YACHENKO O G, et al. Synthesis, structure, and resistivity properties of K1− xBa xNbO3 (0.2 ≤ x ≤ 0.5) and K0.5Sr0.5NbO3 [J]. Materials Research Bulletin, 1995, 30(11): 1379–1386. doi: 10.1016/0025-5408(95)00117-4 [74] CASAIS M T, ALONSO J A, RASINES I, et al. Preparation, neutron structural study and characterization of BaNbO3: a Pauli-like metallic perovskite [J]. Materials Research Bulletin, 1995, 30(2): 201–208. [75] BRIXNER L H. X-ray study and electrical properties of system Ba xSr1− xMoO3 [J]. Journal of Inorganic and Nuclear Chemistry, 1960, 14(3/4): 225–230. [76] SCHOLDER R, RÄDE D, SCHWARZ H. Über zirkonate, hafnate und thorate von barium, strontium, lithium und natrium [J]. Zeitschrift für Anorganische und Allgemeine Chemie, 1968, 362(3/4): 149–168. [77] MULLER O, WHITE W B, ROY R. Crystal chemistry of some technetium-containing oxides [J]. Journal of Inorganic and Nuclear Chemistry, 1964, 26(12): 2075–2086. doi: 10.1016/0022-1902(64)80152-4 [78] SARKOZY R F, CHAMBERLAND B L. The preparation of several new ternary oxides of osmium [J]. Materials Research Bulletin, 1973, 8(12): 1351–1359. doi: 10.1016/0025-5408(73)90019-6 [79] CHAMBERLAND B L. Solid state preparations and reactions of ternary alkaline-earth osmium oxides [J]. Materials Research Bulletin, 1978, 13(12): 1273–1280. doi: 10.1016/0025-5408(78)90117-4 [80] SHI Y G, GUO Y F, SHIRAKO Y, et al. High-pressure synthesis of 5d cubic perovskite BaOsO3 at 17 GPa: ferromagnetic evolution over 3d to 5d series [J]. Journal of the American Chemical Society, 2013, 135(44): 16507–16516. doi: 10.1021/ja4074408 [81] GALLAGHER P K, JOHNSON JR D W, VOGEL E M, et al. Synthesis and structure of BaPtO3 [J]. Journal of Solid State Chemistry, 1977, 21(4): 277–282. doi: 10.1016/0022-4596(77)90126-8 [82] CASAPU M, GRUNWALDT J D, MACIEJEWSKI M, et al. Enhancement of activity and self-reactivation of NSR-catalysts by temporary formation of BaPtO3-perovskite [J]. Catalysis Letters, 2008, 120(1/2): 1–7. [83] YAMAMOTO T, SHITARA K, KITAGAWA S, et al. Selective hydride occupation in BaVO3− xH x (0.3 ≤ x ≤ 0.8) with face and corner-shared octahedra [J]. Chemistry of Materials, 2018, 30(5): 1566–1574. doi: 10.1021/acs.chemmater.7b04571 [84] YUSA H, SATA N, OHISHI Y. Rhombohedral (9R) and hexagonal (6H) perovskites in barium silicates under high pressure [J]. American Mineralogist, 2007, 92(4): 648–654. [85] HIRAMATSU H, YUSA H, IGARASHI R, et al. An exceptionally narrow band-gap (~4 eV) silicate predicted in the cubic perovskite structure: BaSiO3 [J]. Inorganic Chemistry, 2017, 56(17): 10535–10542. doi: 10.1021/acs.inorgchem.7b01510 [86] 谢亚飞, 姜昌国, 罗兴丽, 等. 6H型六方钙钛矿相BaGeO3 的高温高压合成 [J]. 高压物理学报, 2021, 35(5): 051201. doi: 10.11858/gywlxb.20210761XIE Y F, JIANG C G, LUO X L, et al. Synthesis of 6H-type hexagonal perovskite phase of BaGeO3 at high temperature and high pressure [J]. Chinese Journal of High Pressure Physics, 2021, 35(5): 051201. doi: 10.11858/gywlxb.20210761 [87] LONGO J M, KAFALAS J A. Pressure-induced structural changes in the system Ba1− xSr xRuO3 [J]. Materials Research Bulletin, 1968, 3(8): 687–692. [88] ZHAO J G, YANG L X, YU Y, et al. High-pressure synthesis of orthorhombic SrIrO3 perovskite and its positive magnetoresistance [J]. Journal of Applied Physics, 2008, 103(10): 103706. doi: 10.1063/1.2908879 [89] CAO G, BOLIVAR J, MCCALL S, et al. Weak ferromagnetism, metal-to-nonmetal transition, and negative differential resistivity in single-crystal Sr2IrO4 [J]. Physical Review B, 1998, 57(18): R11039–R11042. doi: 10.1103/PhysRevB.57.R11039 [90] CAO G, XIN Y, ALEXANDER C S, et al. Anomalous magnetic and transport behavior in the magnetic insulator Sr3Ir2O7 [J]. Physical Review B, 2002, 66(21): 214412. doi: 10.1103/PhysRevB.66.214412 -







 下载:
下载: