| [1] |
HIKAMI S, MATSUDA Y. High TC superconductors of the perovskite structure oxides [J]. Japanese Journal of Applied Physics, 1987, 26(Suppl 3-2): 1027. doi: 10.7567/JJAPS.26S3.1027
|
| [2] |
ZHENG T, WU J G, XIAO D Q, et al. Recent development in lead-free perovskite piezoelectric bulk materials [J]. Progress in Materials Science, 2018, 98: 552–624. doi: 10.1016/j.pmatsci.2018.06.002
|
| [3] |
ITOH M, HAMASAKI Y, TAKASHIMA H, et al. Chemical design of a new displacive type ferroelectric [J]. Dalton Transactions, 2022, 51(7): 2610–2630. doi: 10.1039/D1DT03693A
|
| [4] |
WU J, LYNN J W, GLINKA C J, et al. Intergranular giant magnetoresistance in a spontaneously phase separated perovskite oxide [J]. Physical Review Letters, 2005, 94(3): 037201. doi: 10.1103/PhysRevLett.94.037201
|
| [5] |
FU Q X, TANG X L, HUANG B, et al. Recent progress on the long-term stability of perovskite solar cells [J]. Advanced Science, 2018, 5(5): 1700387. doi: 10.1002/advs.201700387
|
| [6] |
HASE I, YANAGISAWA T. Electronic states of valence-skipping compounds [J]. Journal of Physics: Conference Series, 2008, 108: 012011. doi: 10.1088/1742-6596/108/1/012011
|
| [7] |
MATSUURA H, MUKUDA H, MIYAKE K. Valence skipping phenomena, charge Kondo effect, and superconductivity [J]. AAPPS Bulletin, 2022, 32(1): 30. doi: 10.1007/s43673-022-00056-1
|
| [8] |
AZUMA M, HOJO H, OKA K, et al. Functional transition metal perovskite oxides with 6 s2 lone pair activity stabilized by high-pressure synthesis [J]. Annual Review of Materials Research, 2021, 51: 329–349. doi: 10.1146/annurev-matsci-080819-011831
|
| [9] |
SHANNON R D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides [J]. Acta Crystallographica Section A, 1976, 32(5): 751–767. doi: 10.1107/S0567739476001551
|
| [10] |
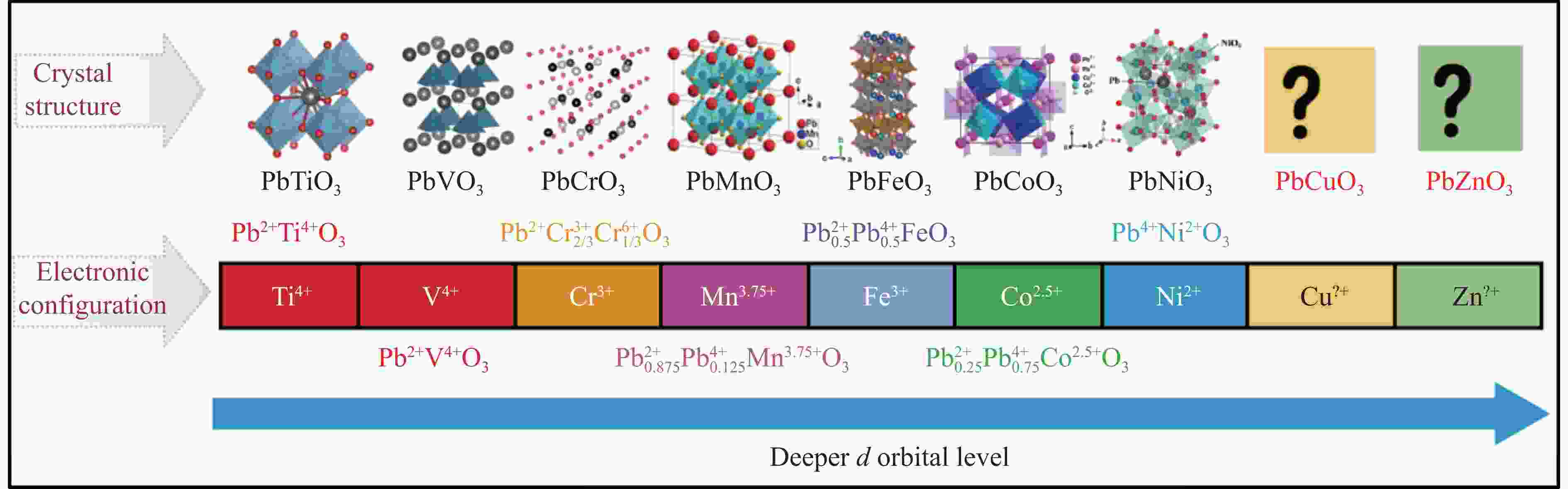
SHPANCHENKO R V, CHERNAYA V V, TSIRLIN A A, et al. Synthesis, structure, and properties of new perovskite PbVO3 [J]. Chemistry of Materials, 2004, 16(17): 3267–3273. doi: 10.1021/cm049310x
|
| [11] |
BELIK A, AZUMA M, SAITO T, et al. Crystallographic features and tetragonal phase stability of PbVO3, a new member of PbTiO3 family [J]. Chemistry of Materials, 2005, 17(2): 269–273. doi: 10.1021/cm048387i
|
| [12] |
LEIST T, GRANZOW T, JO W, et al. Effect of tetragonal distortion on ferroelectric domain switching: a case study on La-doped BiFeO3-PbTiO3 ceramics [J]. Journal of Applied Physics, 2010, 108(1): 014103. doi: 10.1063/1.3445771
|
| [13] |
OKA K, YAMADA I, AZUMA M, et al. Magnetic ground-state of perovskite PbVO3 with large tetragonal distortion [J]. Inorganic Chemistry, 2008, 47(16): 7355–7359. doi: 10.1021/ic800649a
|
| [14] |
PAN Z, NISHIKUBO T, SAKAI Y, et al. Observation of stabilized monoclinic phase as a “bridge” at the morphotropic phase boundary between tetragonal perovskite PbVO3 and rhombohedral BiFeO3 [J]. Chemistry of Materials, 2020, 32(8): 3615–3620. doi: 10.1021/acs.chemmater.0c00944
|
| [15] |
YAMAMOTO H, IMAI T, SAKAI Y, et al. Colossal negative thermal expansion in electron-doped PbVO3 perovskites [J]. Angewandte Chemie International Edition 2018, 57(27): 8170−8173.
|
| [16] |
ROTH W L, DEVRIES R C. Crystal and magnetic structure of PbCrO3 [J]. Journal of Applied Physics, 1967, 38(3): 951–952. doi: 10.1063/1.1709698
|
| [17] |
CHAMBERLAND B L, MOELLER C W. A study on the PbCrO3 perovskite [J]. Journal of Solid State Chemistry, 1972, 5(1): 39–41. doi: 10.1016/0022-4596(72)90006-0
|
| [18] |
DEVRIES R C, ROTH W L. High-pressure synthesis of PbCrO3 [J]. Journal of the American Ceramic Society, 1968, 51(2): 72–75. doi: 10.1111/j.1151-2916.1968.tb11839.x
|
| [19] |
XIAO W S, TAN D Y, XIONG X L, et al. Large volume collapse observed in the phase transition in cubic PbCrO3 perovskite [J]. Proceedings of the National Academy of Sciences the United States of America, 2010, 107(32): 14026–14029. doi: 10.1073/pnas.1005307107
|
| [20] |
AREVALO-LOPEZ Á M, ALARIO-FRANCO M Á. On the structure and microstructure of “PbCrO3” [J]. Journal of Solid State Chemistry, 2007, 180(11): 3271–3279. doi: 10.1016/j.jssc.2007.09.017
|
| [21] |
WU M, ZHENG L R, CHU S Q, et al. Pressure-induced valence change and semiconductor-metal transition in PbCrO3 [J]. The Journal of Physical Chemistry C, 2014, 118(40): 23274–23278. doi: 10.1021/jp5072346
|
| [22] |
YU R Z, HOJO H, WATANUKI M, et al. Melting of Pb charge glass and simultaneous Pb-Cr charge transfer in PbCrO3 as the origin of volume collapse [J]. Journal of the American Chemical Society, 2015, 137(39): 12719–12728. doi: 10.1021/jacs.5b08216
|
| [23] |
CHENG J G, KWEON K E, LARREGOLA S A, et al. Charge disproportionation and the pressure-induced insulator-metal transition in cubic perovskite PbCrO3 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(6): 1670–1674. doi: 10.1073/pnas.1424431112
|
| [24] |
ZHAO J F, HAW S C, WANG X, et al. Stability of the Pb divalent state in insulating and metallic PbCrO3 [J]. Physical Review B, 2023, 107(2): 024107. doi: 10.1103/PhysRevB.107.024107
|
| [25] |
WANG S M, ZHU J L, ZHANG Y, et al. Unusual Mott transition in multiferroic PbCrO3 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(50): 15320–15325. doi: 10.1073/pnas.1510415112
|
| [26] |
WANG S M, CHEN J, WU L S, et al. Giant viscoelasticity near Mott criticality in PbCrO3 with large lattice anomalies [J]. Physical Review Letters, 2022, 128(9): 095702. doi: 10.1103/PhysRevLett.128.095702
|
| [27] |
HAN Y X, WANG S M, LIU Y J, et al. Synthesis of single-crystal perovskite PbCrO3 through a new reaction route at high pressure [J]. High Pressure Research, 2018, 38(2): 136–144. doi: 10.1080/08957959.2018.1428319
|
| [28] |
BOUGEROL C, GORIUS M F, GREY I E. PbMnO2.75—a high-pressure phase having a new type of crystallographic shear structure derived from perovskite [J]. Journal of Solid State Chemistry, 2002, 169(1): 131–138. doi: 10.1016/S0022-4596(02)00065-8
|
| [29] |
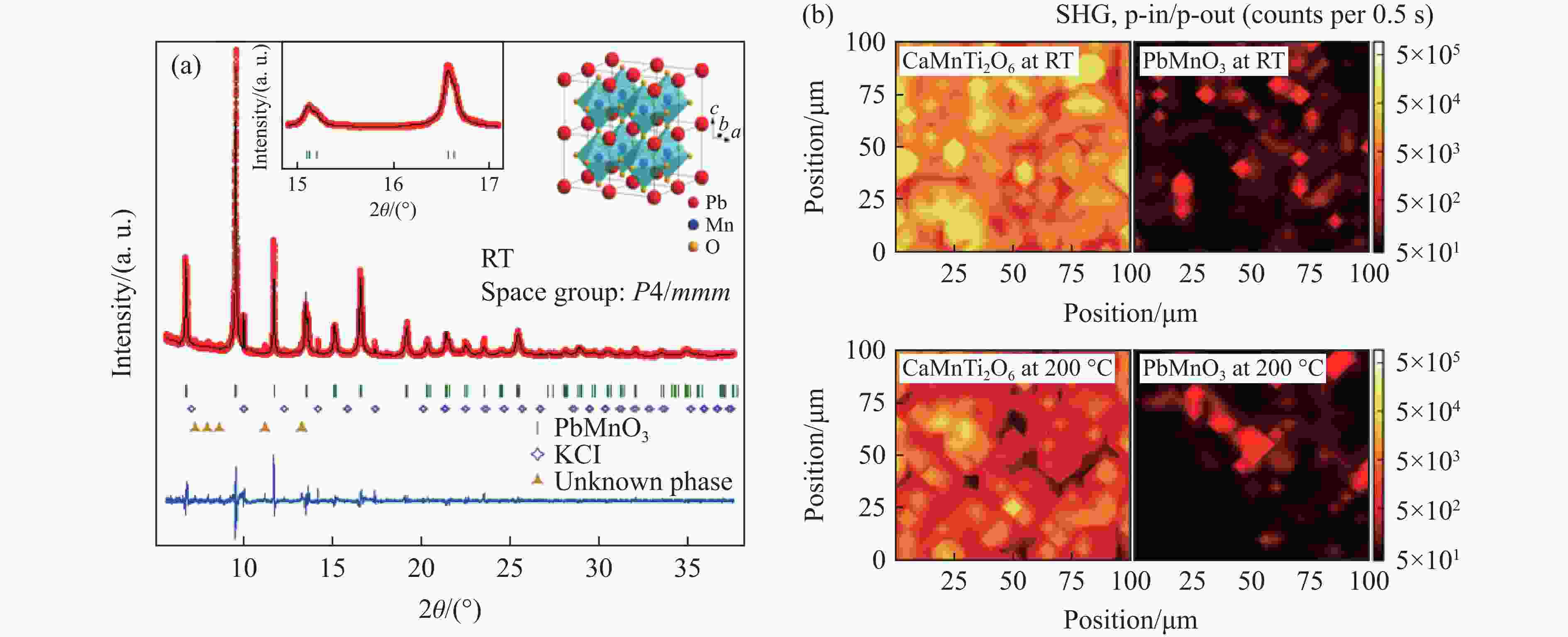
OKA K, AZUMA M, HIRAI S, et al. Pressure-induced transformation of 6H hexagonal to 3C perovskite structure in PbMnO3 [J]. Inorganic Chemistry, 2009, 48(5): 2285–2288. doi: 10.1021/ic802081f
|
| [30] |
CHMAISSEM O, DABROWSKI B, KOLESNIK S, et al. Relationship between structural parameters and the Néel temperature in Sr1– x Ca x MnO3(0<~ x<~1) and Sr1– y Ba y MnO3 ( y<~0.2) [J]. Physical Review B, 2001, 64(13): 134412. doi: 10.1103/PhysRevB.64.134412
|
| [31] |
WOLLAN E O, KOFHLER W C. Neutron diffraction study of the magnetic properties of the series of perovskite-type compounds [(1– x)La, xCa]MnO3 [J]. Physical Review Journals Archive, 1955, 100(2): 545. doi: 10.1103/PhysRev.100.545
|
| [32] |
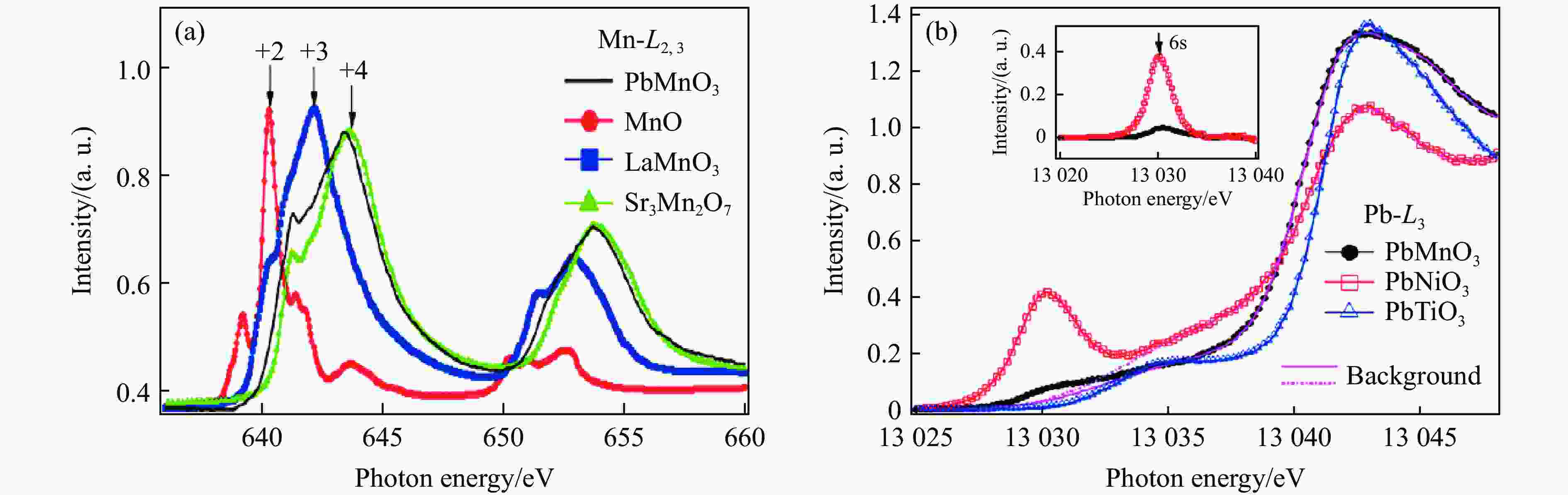
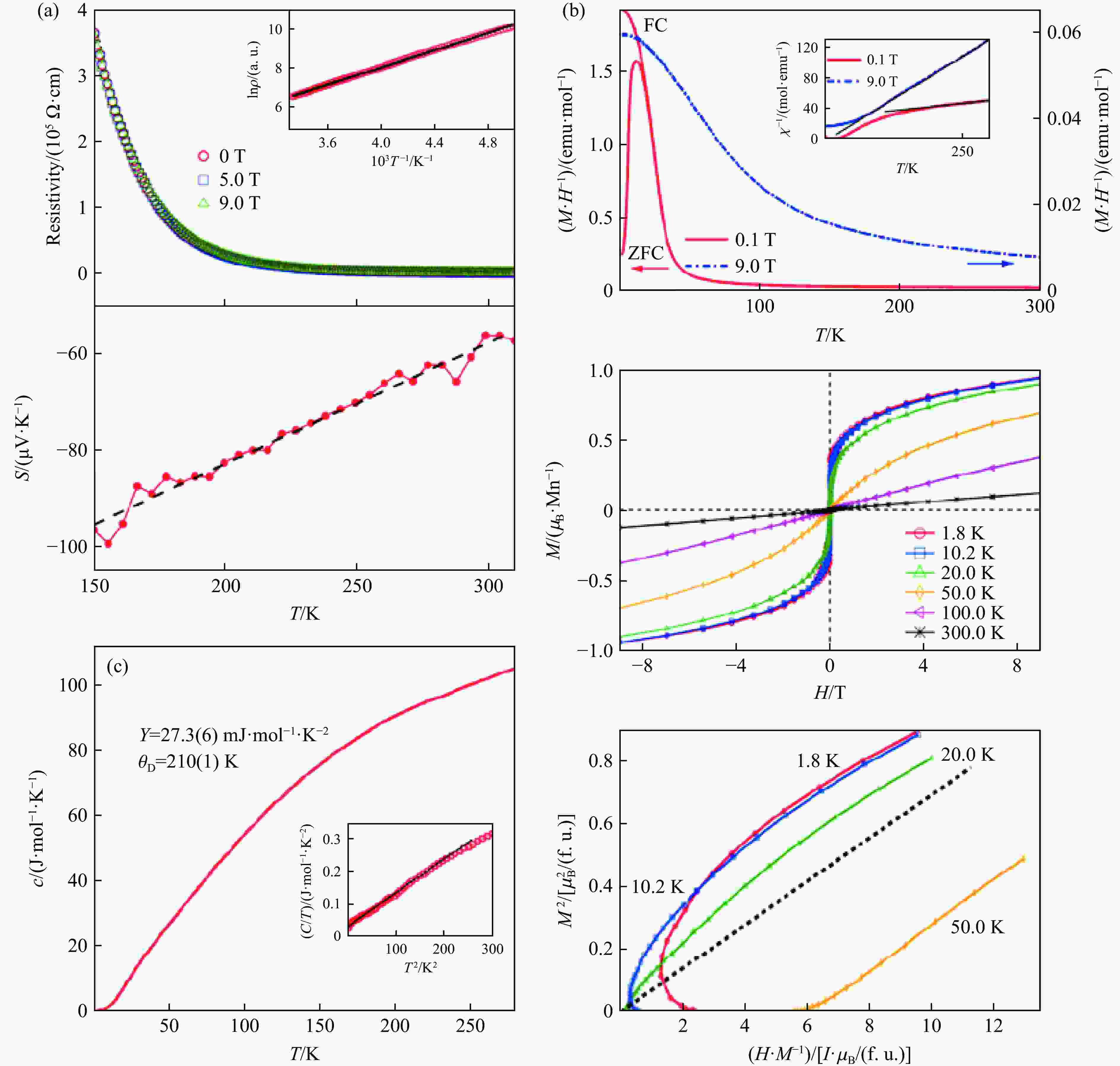
LI X, HU Z W, CHO Y, et al. Charge disproportionation and complex magnetism in a PbMnO3 perovskite synthesized under high pressure [J]. Chemistry of Materials, 2021, 33(1): 92–101. doi: 10.1021/acs.chemmater.0c02706
|
| [33] |
TSUCHIYA T, SAITO S, YOSHIDA M, et al. High-pressure synthesis of a novel PbFeO3 [J]. MRS Online Proceedings Library, 2006, 988: 9880916. doi: 10.1557/PROC-988-0988-QQ09-16
|
| [34] |
WHITE R L. Review of recent work on the magnetic and spectroscopic properties of the rare-earth orthoferrites [J]. Journal of Applied Physics, 1969, 40(3): 1061–1069. doi: 10.1063/1.1657530
|
| [35] |
LI E Y, FENG Z J, KANG B J, et al. Spin switching in single crystal PrFeO3 and spin configuration diagram of rare earth orthoferrites [J]. Journal of Alloys and Compounds, 2019, 811: 152043. doi: 10.1016/j.jallcom.2019.152043
|
| [36] |
ZHAO H J, ÍÑIGUEZ J, CHEN X M, et al. Origin of the magnetization and compensation temperature in rare-earth orthoferrites and orthochromates [J]. Physical Review B, 2016, 93(1): 014417. doi: 10.1103/PhysRevB.93.014417
|
| [37] |
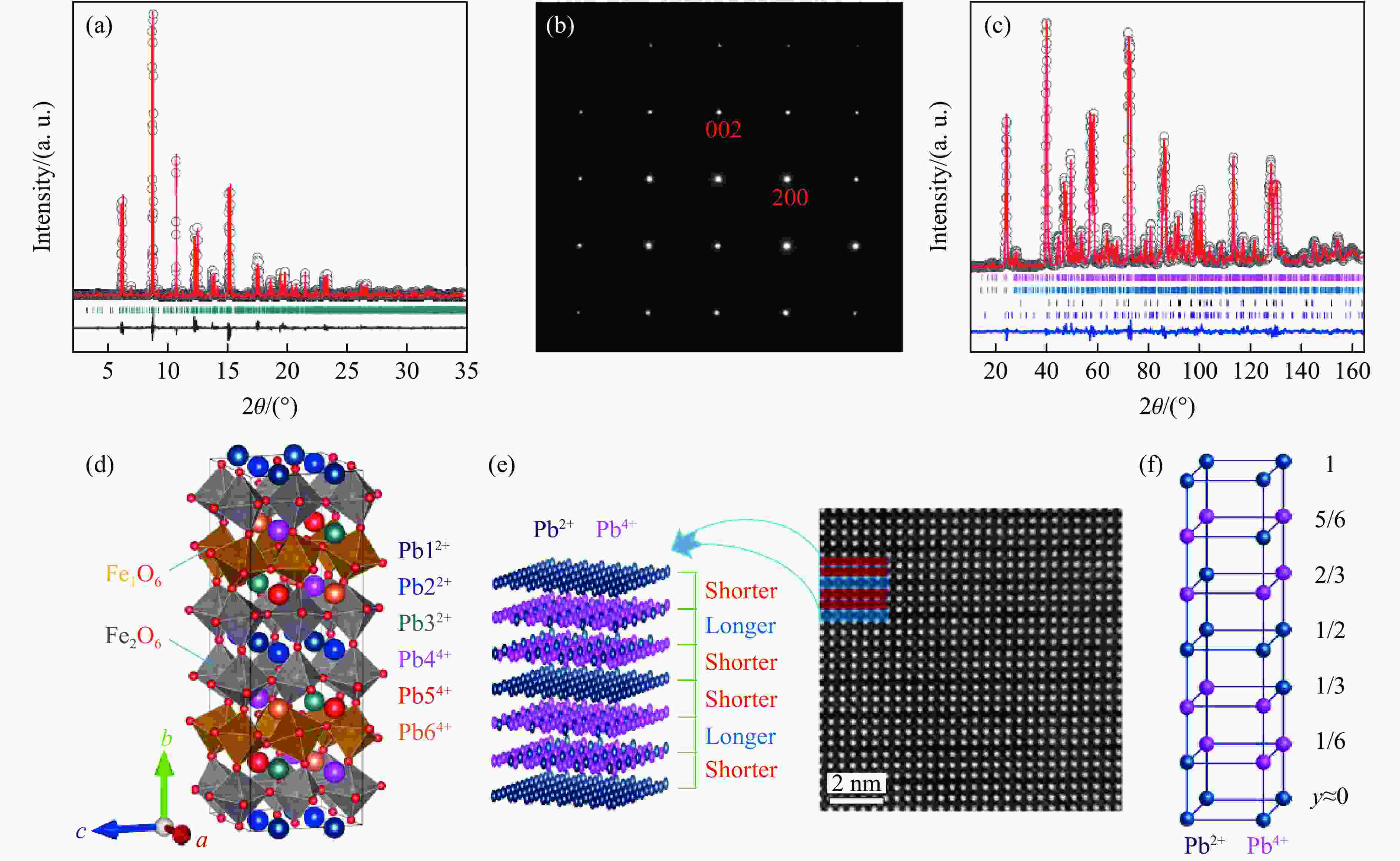
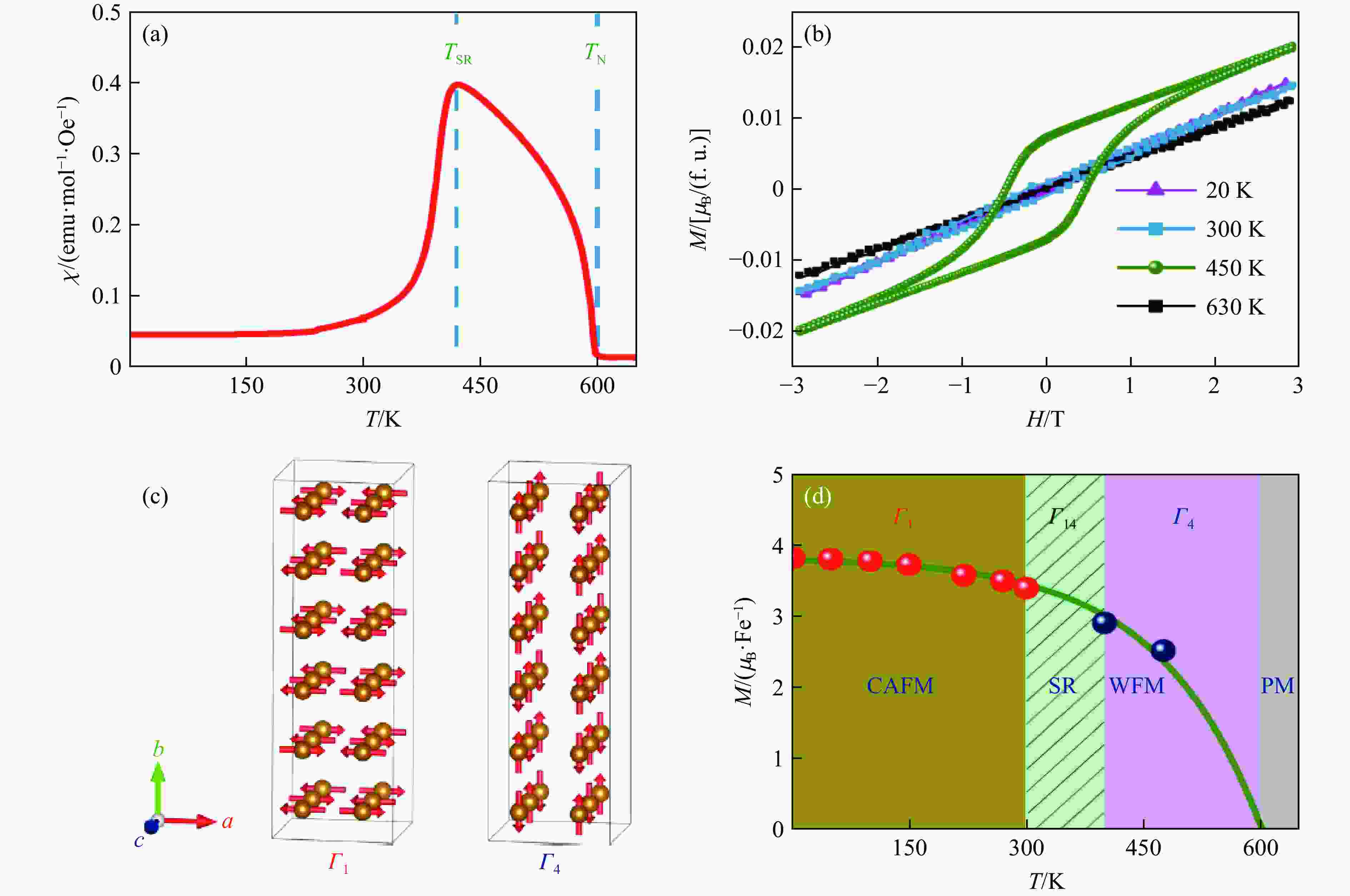
YE X B, ZHAO J F, DAS S, et al. Observation of novel charge ordering and spin reorientation in perovskite oxide PbFeO3 [J]. Nature Communications, 2021, 12: 1917. doi: 10.1038/s41467-021-22064-9
|
| [38] |
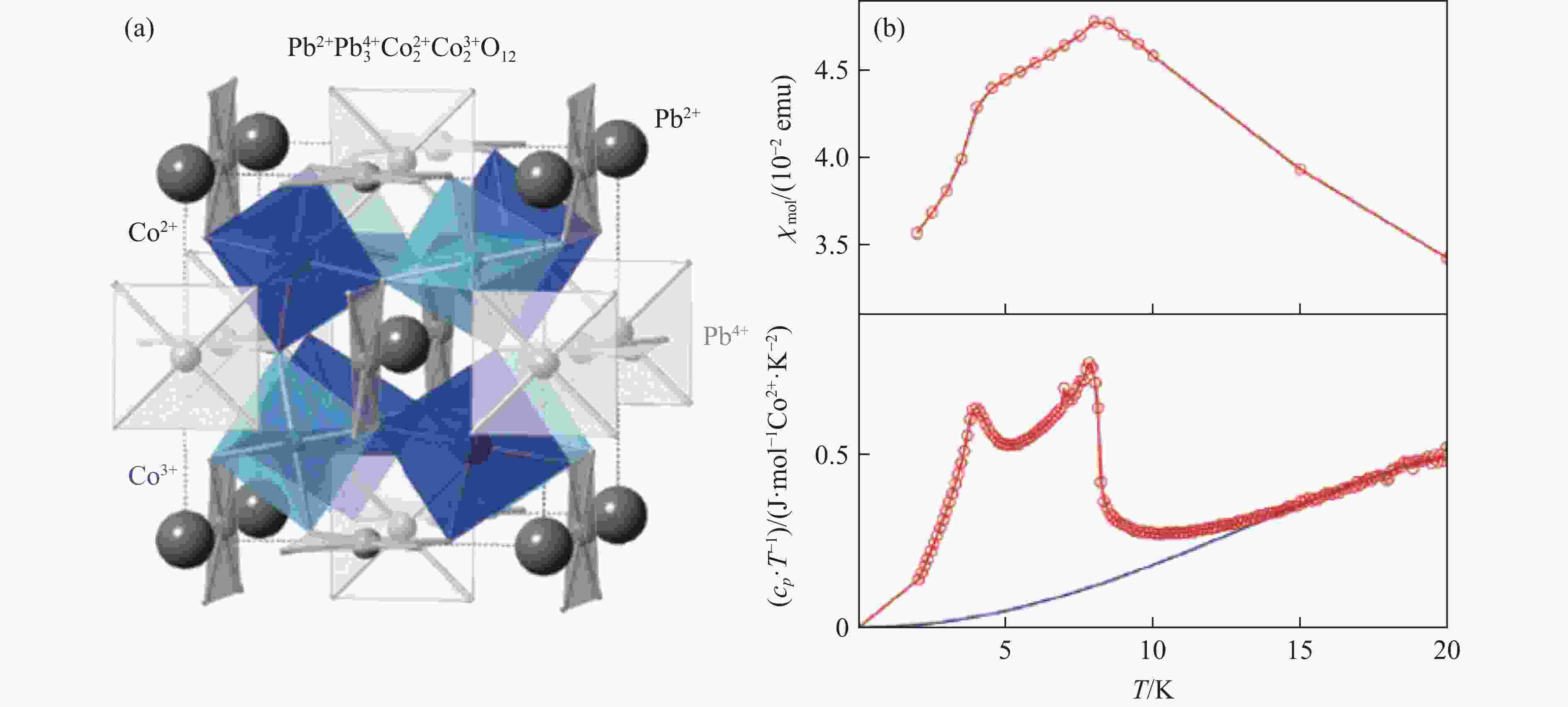
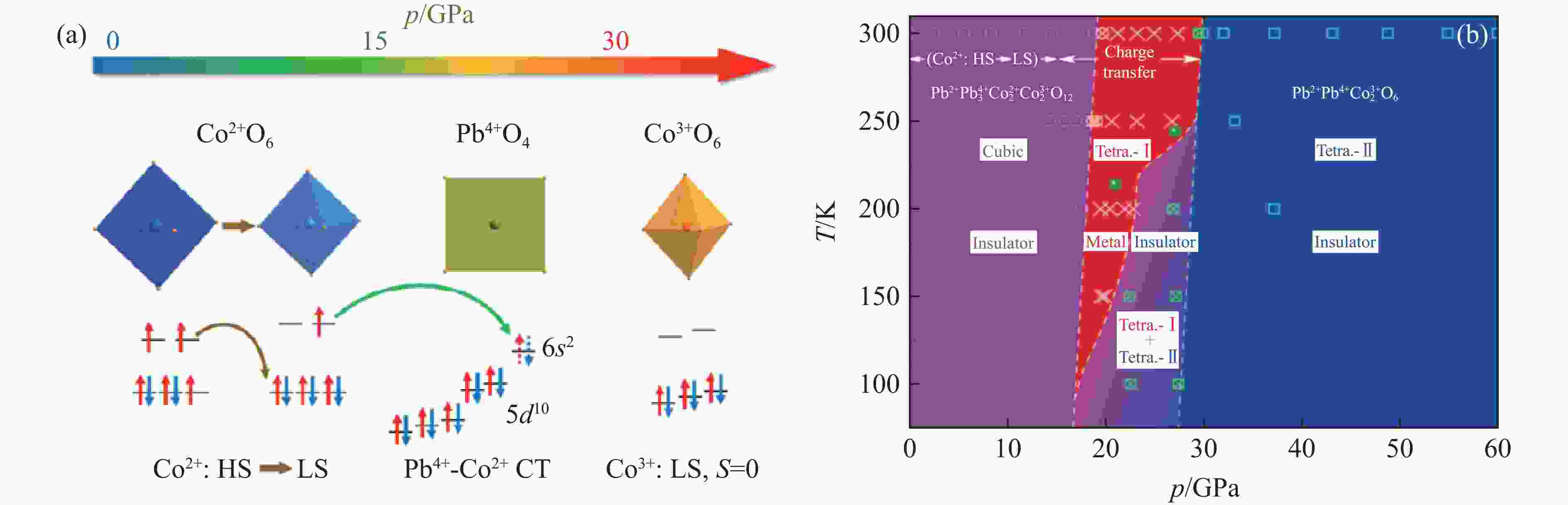
SAKAI Y, YANG J Y, YIN Y Z, et al. A-site and B-site charge orderings in an s-d level controlled perovskite oxide PbCoO3 [J]. Journal of the American Chemical Society, 2017, 139(12): 4574–4581. doi: 10.1021/jacs.7b01851
|
| [39] |
LIU Z H, SAKAI Y, YANG J Y, et al. Sequential spin state transition and intermetallic charge transfer in PbCoO3 [J]. Journal of the American Chemical Society, 2020, 142(12): 5731–5741. doi: 10.1021/jacs.9b13508
|
| [40] |
INAGUMA Y, TANAKA K, TSUCHIYA T, et al. Synthesis, structural transformation, thermal stability, valence state, and magnetic and electronic properties of PbNiO3 with perovskite- and LiNbO3-Type Structures [J]. Journal of the American Chemical Society, 2011, 133(42): 16920–16929. doi: 10.1021/ja206247j
|
| [41] |
YU R Z, HOJO H, MOZOGUCHI T, et al. A new LiNbO3-type polar oxide with closed-shell cations: ZnPbO3 [J]. Journal of Applied Physics, 2015, 118: 094103. doi: 10.1063/1.4930034
|
| [42] |
YANG J Y, DAI J H, LIU ZH, et al. High-pressure synthesis of the cobalt pyrochlore oxide Pb2Co2O7 with large cation mixed occupancy [J]. Inorganic Chemistry, 2017, 56(19): 11676–11680. doi: 10.1021/acs.inorgchem.7b01646
|







 下载:
下载: