Mechanism of Pressure and Carbon Content Regulating Physical Properties of BCxO Compounds
-
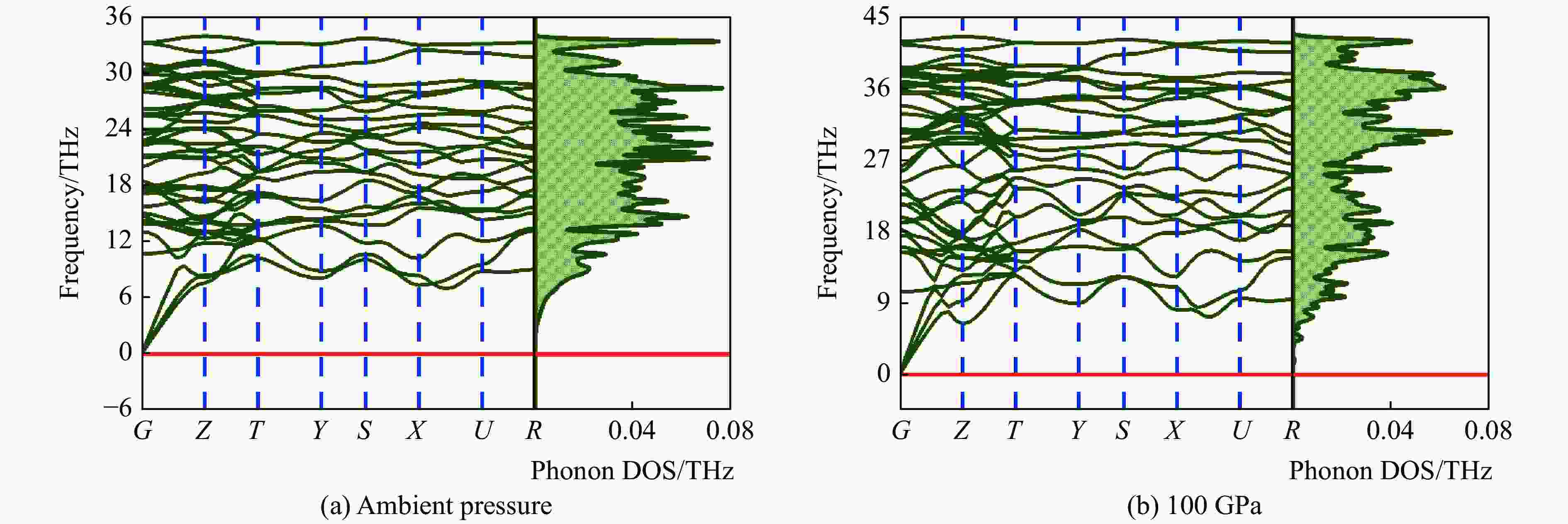
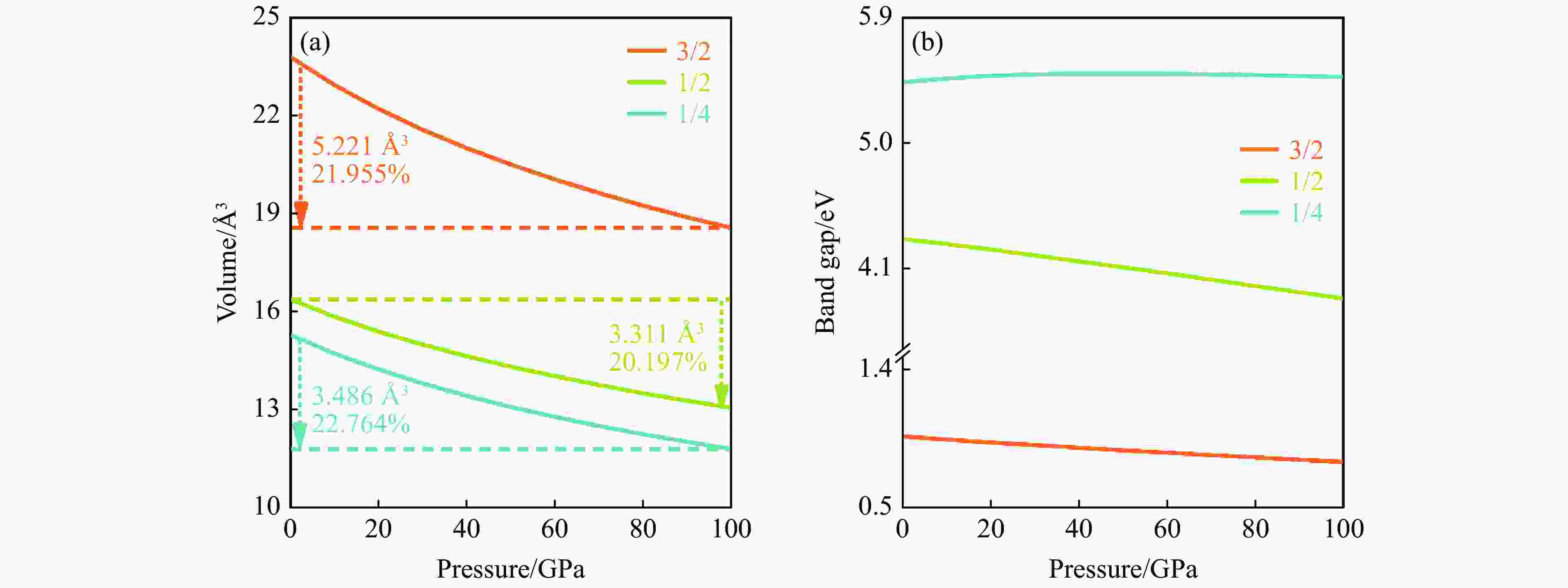
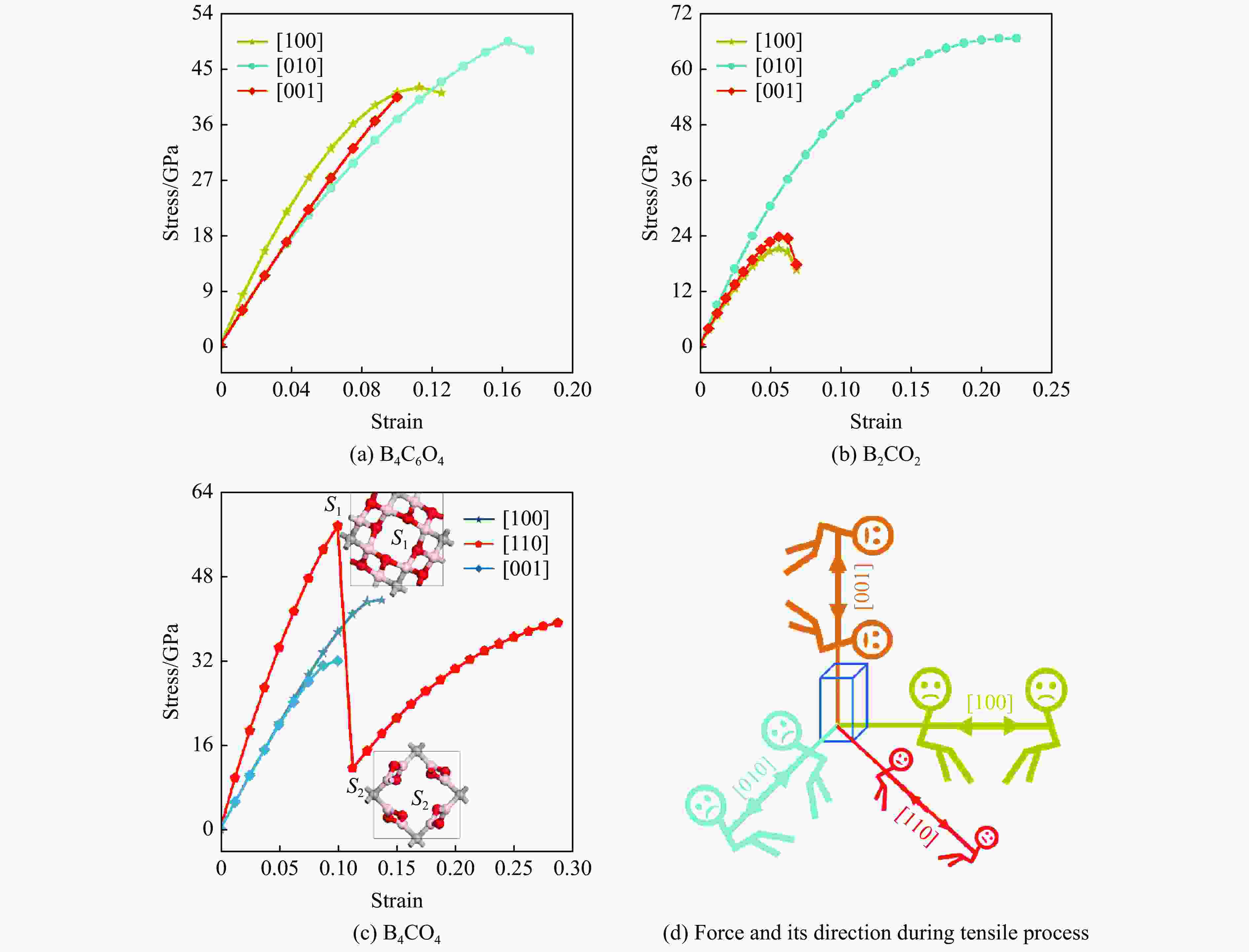
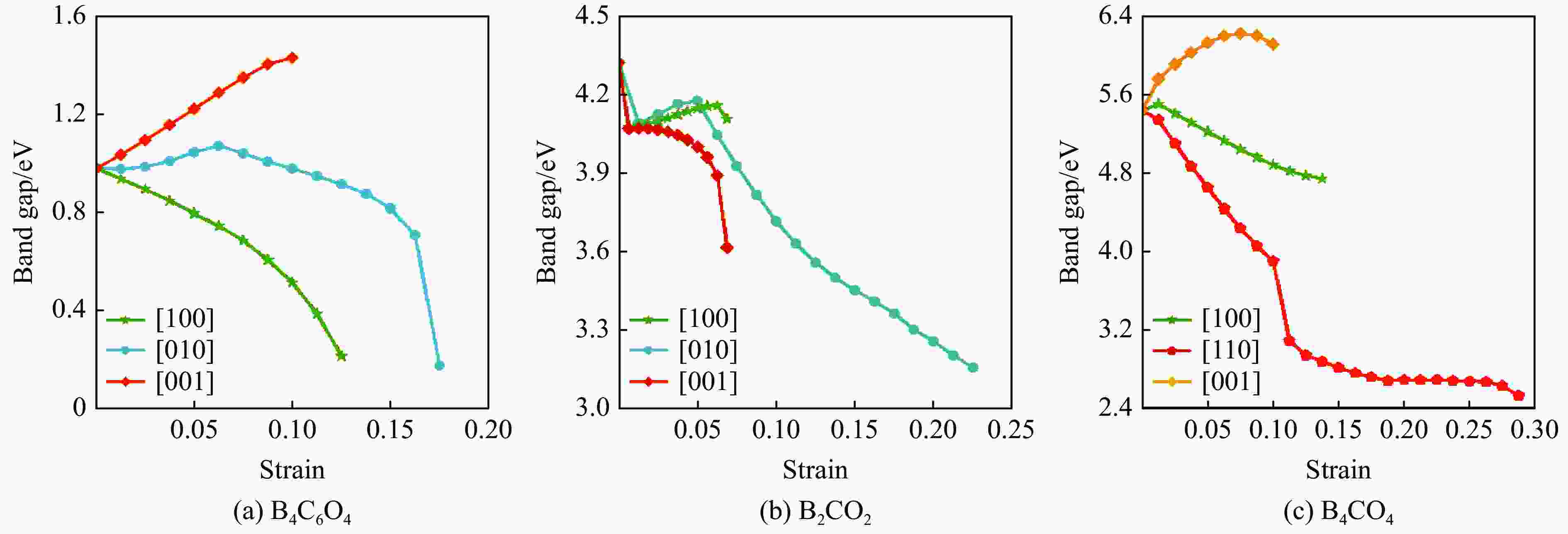
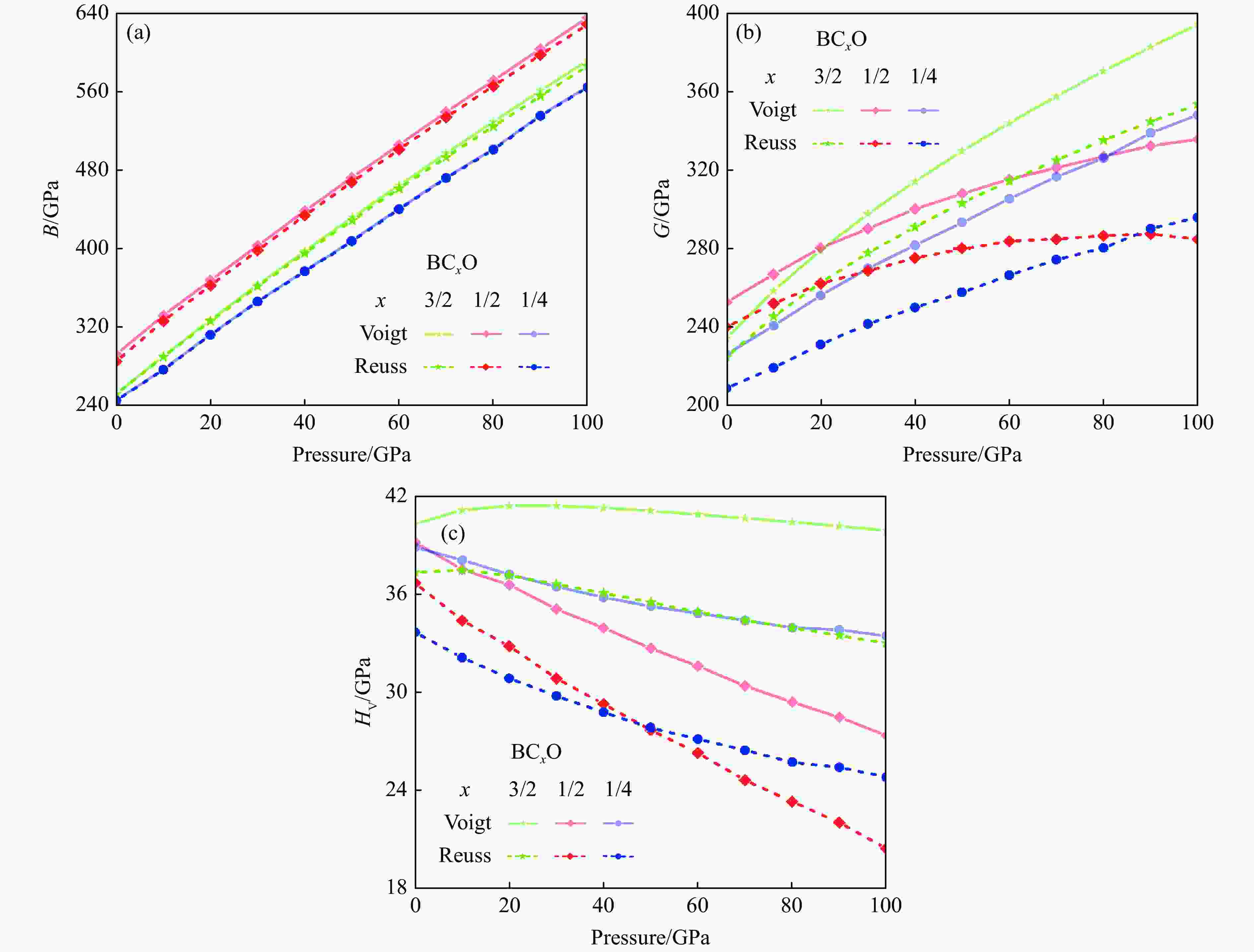
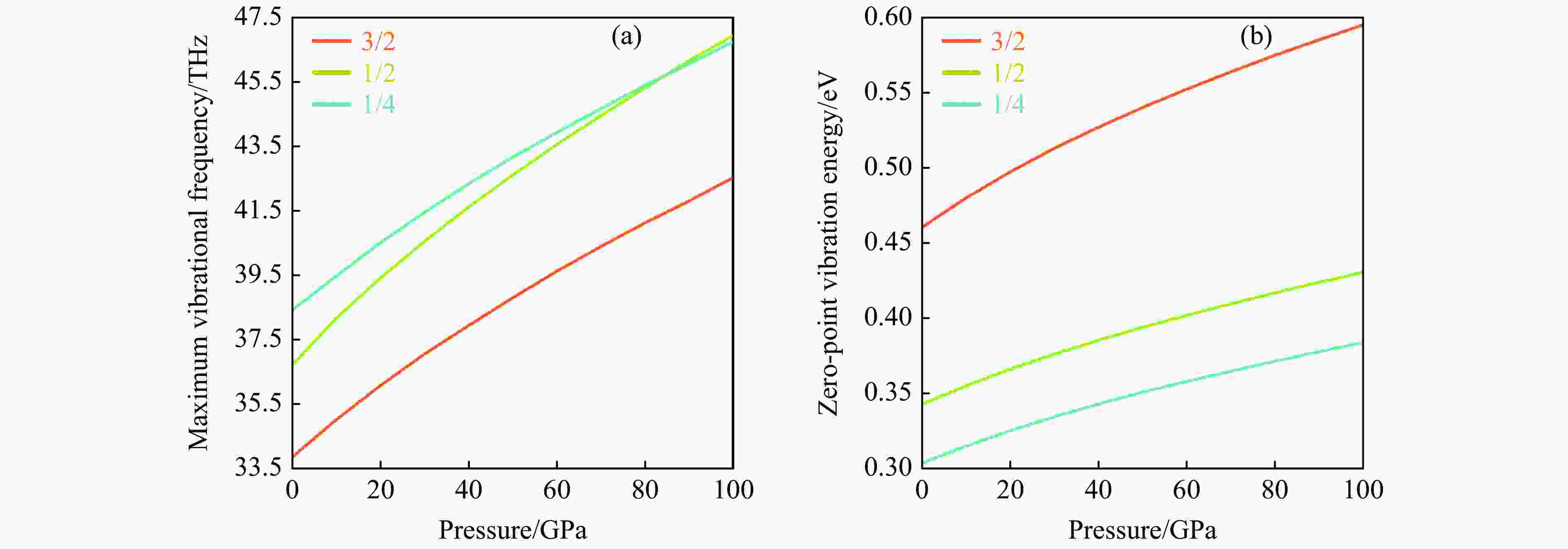
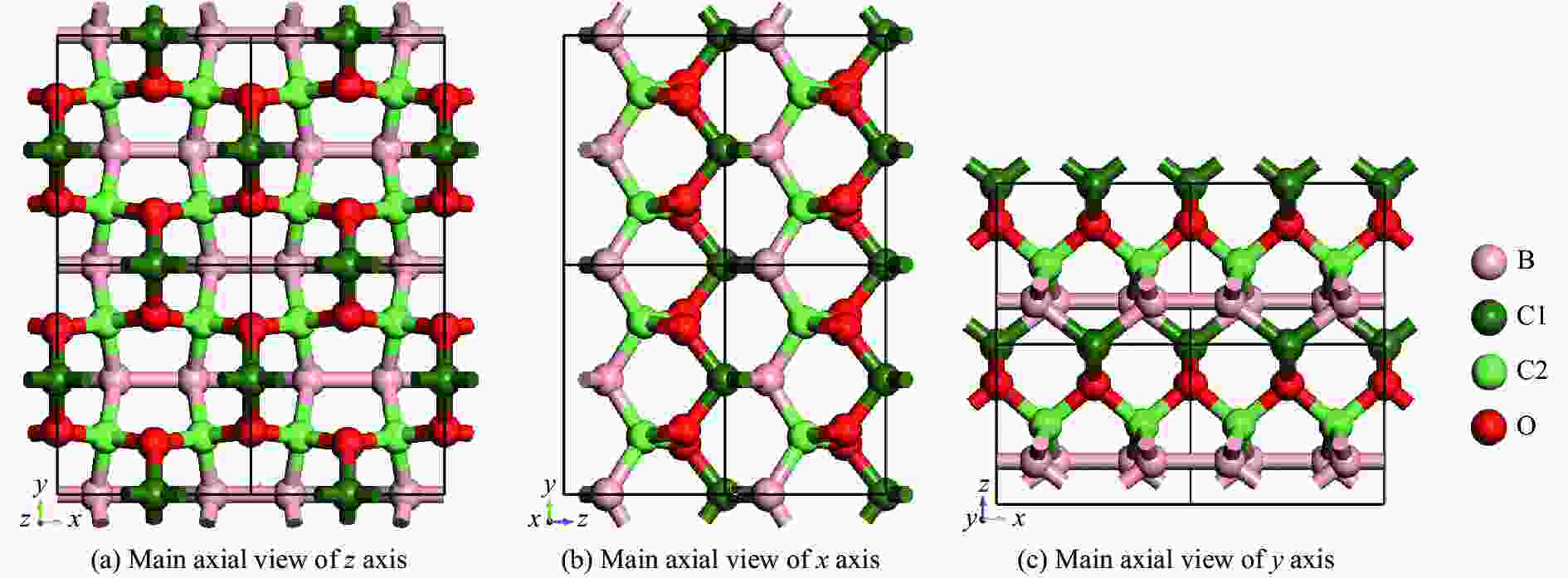
摘要: 结合粒子群优化算法生成的候选结构和第一性原理的稳定性分析,预测出新型B-C-O化合物B4C6O4。B4C6O4具有带隙宽度约2.25 eV的直接带隙半导体属性。研究同属BCxO系列,且结构具有相似性的B4C6O4、B2CO2和B4CO4,发现C含量的降低会导致体系带隙增大,三者的分子式体积随C含量的降低而降低,且100 GPa的高压对三者体积均形成高达20%的压缩。高压导致B2CO2和B4C6O4的带隙持续降低,而B4CO4的带隙先升后降。应力-应变模拟结果表明,3种BCxO(x = 3/2, 1/2, 1/4)化合物均具有较高的极限拉伸应力,同时应力引起的应变会影响3种BCxO化合物的带隙。力学性能研究表明,3种BCxO化合物均具有高弹性模量和高硬度等特点。常压下BCxO的最高声子振动频率均高于30 THz,且由高到低分别为B4CO4、B2CO2、B4C6O4,压力作用使该体系结构的键能持续增强。Abstract: A novel B-C-O compound, B4C6O4, was predicted by combining the candidate structure generated by the particle swarm optimization algorithm and first-principles stability analysis. B4C6O4 has a direct bandgap semiconductivity characteristic with a bandgap width of about 2.25 eV. B4C6O4, B2CO2 and B4CO4 have similar structures and belong to the BCxO series. It was found that the decrease of carbon content led to the increase of the band gap of the system, and the molecular formula volume decreased synchronically with the decrease of carbon content, and the high pressure of 100 GPa compressed the volume of the three as high as 20%. The band gaps of B2CO2 and B4C6O4 continue to decrease due to the effect of high pressure, while the band gap of B4CO4 rise first and then fall. The stress-strain simulation results showed that the three BCxO compounds (x = 3/2, 1/2, 1/4) all have high ultimate tensile stress, and the strain would affect the band gaps of the three BCxO compounds. The mechanical properties of three BCxO compounds showed that they all had high modulus of elasticity and hardness. The highest phonon vibration frequencies of BCxO under chamber pressure are higher than 30 THz, and the relationship is B4CO4 > B2CO2 > B4C6O4. The effect of high pressure will cause the continuous enhancement of the bond energy of the system.
-
Key words:
- high pressure /
- carbon content /
- stability /
- mechanical properties /
- electrical properties
-
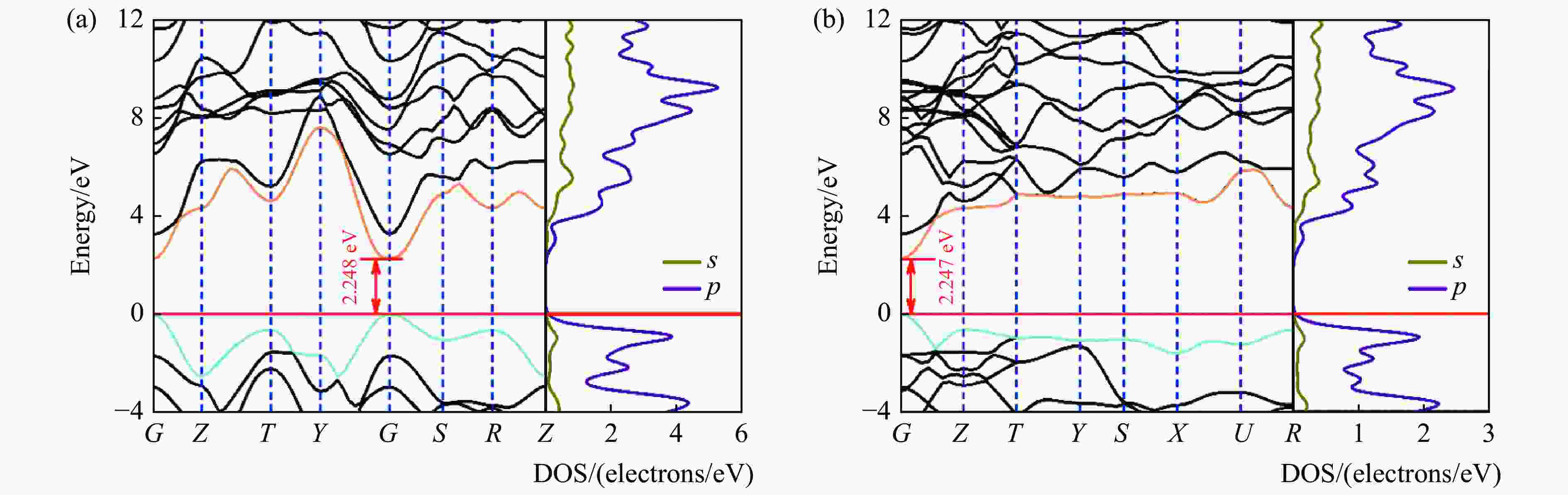
图 4 基于HSE06计算得到的常压下B4C6O4的电子能带结构和态密度:(a)原胞,(b)单胞(水平红线、暗青色曲线和金色曲线分别代表费米能级、VBM和CBM)
Figure 4. Calculated electronic band structures and density of states of B4C6O4 phases via HSE06 with primitive cell (a) and unit cell (b) at ambient pressure (The horizontal red line, dark cyan curve and gold curve represent the Fermi energy level, VBM and CBM, respectively.)
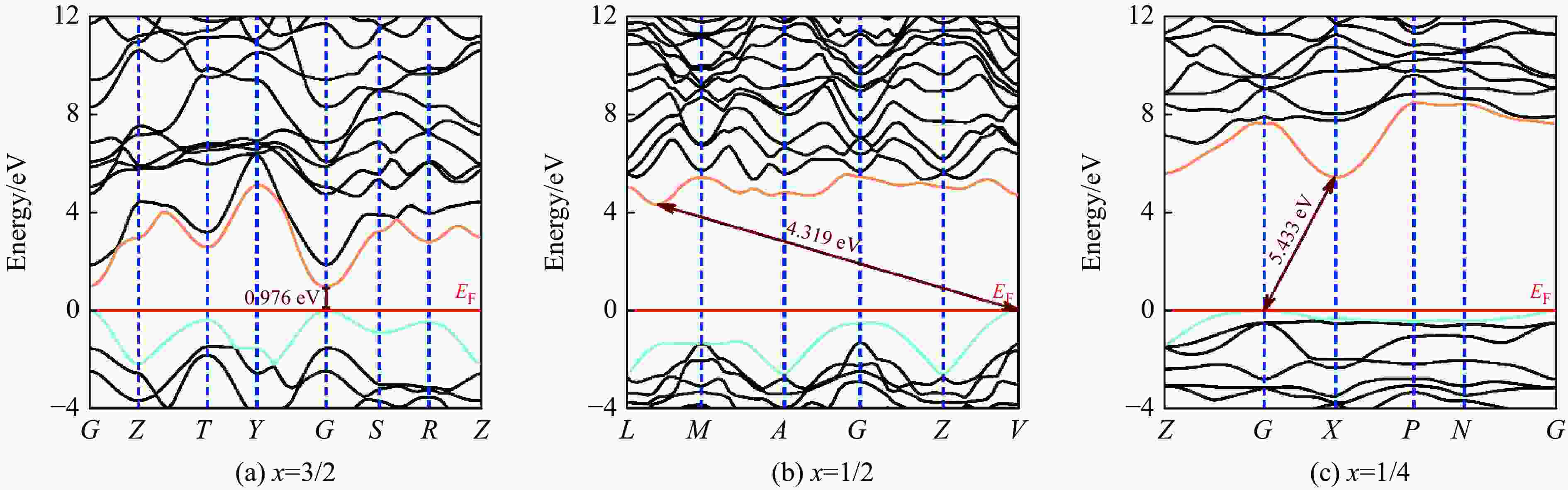
图 5 基于PBE计算常压下BCxO原胞结构的电子能带结构和态密度(水平红线、暗青色曲线和金色曲线分别代表费米能级EF、VBM和CBM)
Figure 5. Calculated electronic band structures and density of states of three BCxO phases with primitive cell via PBE at ambient pressure (The horizontal red line, dark cyan curve and gold curve represent the Fermi energy level EF, VBM and CBM, respectively.)
表 1 常压下B4C6O4的原子坐标
Table 1. Atomic Wyckoff positions of B4C6O4 at ambient pressure
Atom Wyckoff site x y z B 4d 0.299 0.500 0.267 C1 2b 0 0.500 0.991 C2 4c 0.250 0.250 0.491 O 4e 0 0.279 0.746 表 2 常压和100 GPa下B4C6O4的弹性常数
Table 2. Elastic parameters of B4C6O4 at ambient pressure and 100 GPa
GPa Pressure C11 C12 C13 C22 C23 Ambient 693.28 15.78 77.13 542.70 167.35 100 1394.79 146.17 335.20 974.72 497.69 Pressure C33 C44 C55 C66 Ambient 516.07 255.45 197.31 222.21 100 1000.58 517.68 363.80 292.39 表 3 恒温常压条件下B4C6O4在分子动力学过程中的动态结构信息
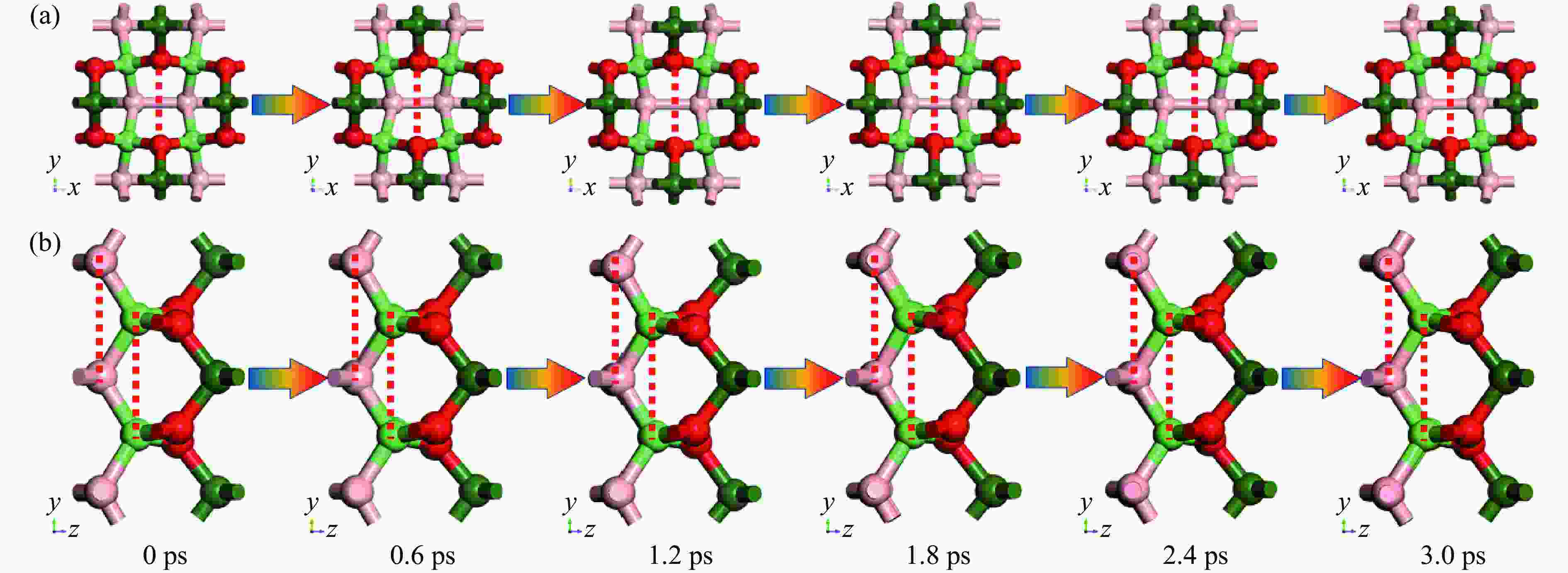
Table 3. Structural information of B4C6O4 during molecular dynamics under constant temperature and ambient pressure
Time/ps $\,\rho $/(g·cm−3) B―B bond length/Å Atom distance/Å C2―C2 O―O B―B 0 3.130 1.842 2.720 3.038 2.757 0.6 3.175 1.776 2.677 3.013 2.775 1.2 3.137 1.869 2.697 3.026 2.722 1.8 3.066 1.827 2.741 3.010 2.810 2.4 3.123 1.846 2.711 3.063 2.806 3.0 3.128 1.884 2.738 3.033 2.798 -
[1] XU B, TIAN Y J. Superhard materials: recent research progress and prospects [J]. Science China Materials, 2015, 58(2): 132–142. doi: 10.1007/s40843-015-0026-5 [2] 刘银娟, 贺端威, 王培, 等. 复合超硬材料的高压合成与研究 [J]. 物理学报, 2017, 66(3): 038103. doi: 10.7498/aps.66.038103LIU Y J, HE D W, WANG P, et al. Syntheses and studies of superhard composites under high pressure [J]. Acta Physica Sinica, 2017, 66(3): 038103. doi: 10.7498/aps.66.038103 [3] KIDALOV S V, SHAKHOV F M, DAVIDENKO V M, et al. Synthesis and properties of superhard crystalline materials in boron-carbon-nitrogen system [J]. Technical Physics Letters, 2011, 37(3): 247–249. doi: 10.1134/S1063785011030266 [4] SOLOZHENKO V L, KURAKEVYCH O O, ANDRAULT D, et al. Ultimate metastable solubility of boron in diamond: synthesis of superhard diamondlike BC5 [J]. Physical Review Letters, 2009, 102(1): 015506. doi: 10.1103/PhysRevLett.102.015506 [5] ZININ P V, MING L C, ISHII H A, et al. Phase transition in BCx system under high-pressure and high-temperature: synthesis of cubic dense BC3 nanostructured phase [J]. Journal of Applied Physics, 2012, 111(11): 114905. doi: 10.1063/1.4723275 [6] KOBAYASHI M, HIGASHI I, BRODHAG C, et al. Structure of B6O boron-suboxide by Rietveld refinement [J]. Journal of Materials Science, 1993, 28(8): 2129–2134. doi: 10.1007/BF00367573 [7] ENDO T, SATO T, SHIMADA M. High-pressure synthesis of B2O with diamond-like structure [J]. Journal of Materials Science Letters, 1987, 6(6): 683–685. doi: 10.1007/BF01770925 [8] ZHAO Y, HE D W, DAEMEN L L, et al. Superhard B-C-N materials synthesized in nanostructured bulks [J]. Journal of Materials Research, 2002, 17(12): 3139–3145. doi: 10.1557/JMR.2002.0454 [9] SOLOZHENKO V L, ANDRAULT D, FIQUET G, et al. Synthesis of superhard cubic BC2N [J]. Applied Physics Letters, 2001, 78(10): 1385–1387. doi: 10.1063/1.1337623 [10] KNITTLE E, KANER R B, JEANLOZ R, et al. High-pressure synthesis, characterization, and equation of state of cubic C-BN solid solutions [J]. Physical Review B, 1995, 51(18): 12149–12156. doi: 10.1103/PhysRevB.51.12149 [11] LIU L Y, HU M, ZHAO Z S, et al. Superhard conductive orthorhombic carbon polymorphs [J]. Carbon, 2020, 158: 546–552. doi: 10.1016/j.carbon.2019.11.024 [12] 李子鹤, 刘超, 马梦东, 等. 新型超硬C5N晶体结构及性能的第一性原理研究 [J]. 高压物理学报, 2018, 32(1): 010103. doi: 10.11858/gywlxb.20170606LI Z H, LIU C, MA M D, et al. Structure and properties of novel superhard C5N: a first-principles study [J]. Chinese Journal of High Pressure Physics, 2018, 32(1): 010103. doi: 10.11858/gywlxb.20170606 [13] LUO X G, GUO X J, XU B, et al. Body-centered superhard BC2N phases from first principles [J]. Physical Review B, 2007, 76(9): 094103. doi: 10.1103/PhysRevB.76.094103 [14] LIU A Y, COHEN M L. Prediction of new low compressibility solids [J]. Science, 1989, 245(4920): 841–842. doi: 10.1126/science.245.4920.841 [15] GARVIE L A J, HUBERT H, PETUSKEY W T, et al. High-pressure, high-temperature syntheses in the B-C-N-O system [J]. Journal of Solid State Chemistry, 1997, 133(2): 365–371. doi: 10.1006/jssc.1997.7583 [16] BOLOTINA N B, DYUZHEVA T I, BENDELIANI N A. Atomic structure of boron suboxycarbide B(C,O)0.155 [J]. Crystallography Reports, 2001, 46(5): 734–740. doi: 10.1134/1.1405858 [17] LI Y W, LI Q, MA Y M. B2CO: a potential superhard material in the B-C-O system [J]. EPL (Europhysics Letters), 2011, 95(6): 66006. doi: 10.1209/0295-5075/95/66006 [18] ZHANG M G, YAN H Y, ZHENG B B, et al. Influences of carbon concentration on crystal structures and ideal strengths of B2CxO compounds in the B-C-O system [J]. Scientific Reports, 2015, 5: 15481. doi: 10.1038/srep15481 [19] LIU C, ZHAO Z S, LUO K, et al. Superhard orthorhombic phase of B2CO compound [J]. Diamond and Related Materials, 2017, 73: 87–92. doi: 10.1016/j.diamond.2016.07.010 [20] QIAO L P, JIN Z, YAN G Y, et al. Density-functional-studying of oP8-, tI16-, and tP4-B2CO physical properties under pressure [J]. Journal of Solid State Chemistry, 2019, 270: 642–650. doi: 10.1016/j.jssc.2018.12.012 [21] LIU C, CHEN M W, HE J L, et al. Superhard B2CO phases derived from carbon allotropes [J]. RSC Advances, 2017, 7(82): 52192–52199. doi: 10.1039/c7ra09277f [22] YAN H Y, ZHANG M G, WEI Q, et al. A new orthorhombic ground-state phase and mechanical strengths of ternary B2CO compound [J]. Chemical Physics Letters, 2018, 701: 86–92. doi: 10.1016/j.cplett.2018.04.041 [23] CHEN M W, LIU C, LIU M L, et al. Exploring the electronic, mechanical, and anisotropy properties of novel tetragonal B2CO phase [J]. Journal of Materials Research, 2019, 34(21): 3617–3626. doi: 10.1557/jmr.2019.271 [24] WANG S N, OGANOV A R, QIAN G R, et al. Novel superhard B-C-O phases predicted from first principles [J]. Physical Chemistry Chemical Physics, 2016, 18(3): 1859–1863. doi: 10.1039/c5cp05367f [25] NURUZZAMAN M, ALAM M A, SHAH M A H, et al. Investigation of thermodynamic stability, mechanical and electronic properties of superhard tetragonal B4CO4 compound: ab initio calculations [J]. Computational Condensed Matter, 2017, 12: 1–8. doi: 10.1016/j.cocom.2017.05.005 [26] ZHENG B B, ZHANG M G, WANG C J. Exploring the mechanical anisotropy and ideal strengths of tetragonal B4CO4 [J]. Materials, 2017, 10(2): 128. doi: 10.3390/ma10020128 [27] QIAO L P, JIN Z. Two B-C-O compounds: structural, mechanical anisotropy and electronic properties under pressure [J]. Materials, 2017, 10(12): 1413. doi: 10.3390/ma10121413 [28] LIU C, CHEN M W, YANG Y, et al. Theoretical exploring the mechanical and electrical properties of tI12-B6C4O2 [J]. Computational Materials Science, 2018, 150: 259–264. doi: 10.1016/j.commatsci.2018.04.020 [29] 刘超, 陈明伟, 梁彤祥. B-C-O化合物硬质结构的理论设计与性质研究[M]. 北京: 冶金工业出版社, 2020. [30] WANG Y C, LV J, ZHU L, et al. Crystal structure prediction via particle-swarm optimization [J]. Physical Review B, 2010, 82(9): 094116. doi: 10.1103/PhysRevB.82.094116 [31] WANG Y C, LV J, ZHU L, et al. CALYPSO: a method for crystal structure prediction [J]. Computer Physics Communications, 2012, 183(10): 2063–2070. doi: 10.1016/j.cpc.2012.05.008 [32] WANG H, WANG Y C, LV J, et al. CALYPSO structure prediction method and its wide application [J]. Computational Materials Science, 2016, 112: 406–415. doi: 10.1016/j.commatsci.2015.09.037 [33] CLARK S J, SEGALL M D, PICKARD C J, et al. First principles methods using CASTEP [J]. Zeitschrift für Kristallographie-Crystalline Materials, 2005, 220(5/6): 567–570. doi: 10.1524/zkri.220.5.567.65075 [34] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865 [35] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism [J]. Physical Review B, 1990, 41(11): 7892–7895. doi: 10.1103/PhysRevB.41.7892 [36] PARLINSKI K, LI Z Q, KAWAZOE Y. First-principles determination of the soft mode in cubic ZrO2 [J]. Physical Review Letters, 1997, 78(21): 4063–4066. doi: 10.1103/PhysRevLett.78.4063 [37] MOUHAT F, COUDERT F X. Necessary and sufficient elastic stability conditions in various crystal systems [J]. Physical Review B, 2014, 90(22): 224104. doi: 10.1103/PhysRevB.90.224104 [38] BROQVIST P, ALKAUSKAS A, PASQUARELLO A. Defect levels of dangling bonds in silicon and germanium through hybrid functionals [J]. Physical Review B, 2008, 78(7): 075203. doi: 10.1103/PhysRevB.78.075203 [39] KRUKAU A V, VYDROV O A, IZMAYLOV A F, et al. Influence of the exchange screening parameter on the performance of screened hybrid functionals [J]. Journal of Chemical Physics, 2006, 125(22): 224106. doi: 10.1063/1.2404663 [40] 刘超. AlX化合物结构与性质的第一性原理研究[M]. 北京: 冶金工业出版社, 2020. [41] DIAS R P, SILVERA I F. Observation of the Wigner-Huntington transition to metallic hydrogen [J]. Science, 2017, 355(6326): 715–718. doi: 10.1126/science.aal1579 [42] MA Y M, EREMETS M, OGANOV A R, et al. Transparent dense sodium [J]. Nature, 2009, 458(7235): 182–185. doi: 10.1038/nature07786 [43] WU Z J, ZHAO E J, XIANG H P, et al. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles [J]. Physical Review B, 2007, 76(5): 054115. doi: 10.1103/PhysRevB.76.054115 [44] TIAN Y J, XU B, ZHAO Z S. Microscopic theory of hardness and design of novel superhard crystals [J]. International Journal of Refractory Metals and Hard Materials, 2012, 33: 93–106. doi: 10.1016/j.ijrmhm.2012.02.021 -






 下载:
下载: