Synthesis of Cubic Gauche Nitrogen (cg-N) under High Pressure and High Temperature
-
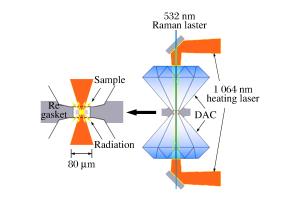
摘要: 以共价N─N单键结合的三维网状聚合氮(cg-N)是一种理想的高能量密度材料。在室温下将分子态氮加压至135.6GPa,观测到了氮的一系列“固体分子态-固体分子态”转变(β-δ-ε-ζ-η);且在不引入任何激光吸收材料的情况下,直接对红色的非晶η氮进行双面金刚石压砧激光加热,在133.9GPa、2000K的条件下成功地合成出透明cg-N,并测量得到cg-N在134GPa附近拉曼A模频率对压力的变化率为1.56cm-1/GPa。
-
关键词:
- 聚合氮 /
- 高能量密度材料(HEDM) /
- 高温高压 /
- 拉曼散射 /
- 激光加热金刚石压砧
Abstract: Three-dimensional polymetric cubic gauche nitrogen (cg-N) combined with covalent N─N single bonds is an ideal high energy density material (HEDM).A series of solid molecular state-to-solid molecular state transitions (β-δ-ε-ζ-η) in nitrogen were observed in experiment upon pressurizing the molecular nitrogen up to 135.6GPa in ambient condition.Under 133.9GPa and at 2000K, the transparent cg-N was successfully synthesized using the double-side laser heating diamond-anvil cell (LHDAC) without any laser absorbing material.In addition, the pressure coefficient of the Raman A mode for cg-N is 1.56cm-1/GPa at about 134GPa. -
图 3 (a) 不同压力下以及激光加热前、后氮的拉曼光谱; (b)第一次激光加热(134.3GPa、约1000K)后样品的光学照片; (c)第二次激光加热(133.9GPa、约2000K)后样品的光学照片; (d)cg-N拉曼A模频率与压力的关系,作为比较,先前报道过的理论与实验值也一同给出
Figure 3. (a)Raman spectra of nitrogen under various pressures and after LHDAC experiments; (b)Optical photograph of nitrogen after the 1st LHDAC experiment (134.3GPa and about 1000K); (c)Optical photograph of nitrogen after the 2nd LHDAC experiment (133.9GPa and about 2000K); (d)Comparison of the relation between Raman shift of A mode of cg-N and applied pressure
-
[1] MCMAHAN A K, LESAR R.Pressure dissociation of solid nitrogen under 1Mbar[J]. Physical Review Letters, 1985, 54(17):1929-1932. doi: 10.1103/PhysRevLett.54.1929 [2] MAILHIOT C, YANG L H, MCMAHAN A K, et al.Polymeric nitrogen[J]. Physical Review B, 1994, 46(22):14419-14435. [3] BARBEE T W.Metastability of atomic phases of nitrogen[J]. Physical Review B, 1993, 48(13):9327-9330. doi: 10.1103/PhysRevB.48.9327 [4] OLIJNYK H, JEPHCOAT A P.Vibrational dynamics of isotopically dilute nitrogen to 104GPa[J]. Physical Review Letters, 1999, 83(2):332-335. doi: 10.1103/PhysRevLett.83.332 [5] GONCHAROV A F, GREGORYANZ E, MAO H K, et al.Optical evidence for a nonmolecular phase of nitrogen above 150GPa[J]. Physical Review Letters, 2000, 85(6):1262-1265. doi: 10.1103/PhysRevLett.85.1262 [6] GREGORYANZ E, GONCHAROV A F, HEMLEY R J, et al.High-pressure amorphous nitrogen[J]. Physical Review B, 2001, 64(5):052103. [7] EREMETS M I, HEMLEY R J, MAO H K, et al.Semiconducting non-molecular nitrogen up to 240GPa and its low-pressure stability[J]. Nature, 2001, 411(6834):170-174. doi: 10.1038/35075531 [8] GONCHAROV A F, GREGORYANZ E, MAO H K, et al.Vibrational dynamics of solid molecular nitrogen to megabar pressures[J]. Low Temperature Physics, 2001, 27(9):866-869. doi: 10.1063/1.1414578 [9] GREGORYANZ E, GONCHAROV A F, HEMLEY R J, et al.Raman, infrared, and X-ray evidence for new phases of nitrogen at high pressures and temperatures[J]. Physical Review B, 2002, 66(22):224108. doi: 10.1103/PhysRevB.66.224108 [10] EREMETS M I, ALEXANDER G, GAVRILIUK I, et al.Single-bonded cubic form of nitrogen[J]. Nature Materials, 2004, 3(8):558-563. doi: 10.1038/nmat1146 [11] EREMETS M I, GAVRILIUK A G, SEREBRYANAYA N R, et al.Structural transformation of molecular nitrogen to a single-bonded atomic state at high pressures[J]. The Journal of Chemical Physics, 2004, 121(22):11296-11300. doi: 10.1063/1.1814074 [12] GONCHAROV A, GREGORYANZ E. Solid nitrogen at extreme conditions of high pressure and temperature: UCRL-BOOK-203536 [R]. Livermore, CA: Lawrence Livermore National Laboratory (LLNL), 2004. [13] LIPP M J, KLEPEIS J P, BAER B J, et al.Transformation of molecular nitrogen to nonmolecular phases at megabar pressures by direct laser heating[J].Physical Review B, 2007, 76(1):014113. doi: 10.1103/PhysRevB.76.014113 [14] GREGORYANZ E, GONCHAROV A F, SANLOUP C, et al.High P-T transformations of nitrogen to 170GPa[J]. The Journal of Chemical Physics, 2007, 126(18):184505. doi: 10.1063/1.2723069 [15] TOMASINO D, KIM M, SMITH J, et al.Pressure-induced symmetry-lowering transition in dense nitrogen to layered polymeric nitrogen (LP-N) with colossal Raman intensity[J]. Physical Review Letters, 2014, 113(20):205502. doi: 10.1103/PhysRevLett.113.205502 [16] AKAHAMA Y, KAWAMURA H.High-pressure Raman spectroscopy of diamond anvils to 250GPa:method for pressure determination in the multimegabar pressure range[J]. Journal of Applied Physics, 2004, 96(7):3748-3751. doi: 10.1063/1.1778482 [17] AKAHAMA Y, KAWAMURA H.Pressure calibration of diamond anvil Raman gauge to 310GPa[J]. Journal of Applied Physics, 2006, 100(4):043516. doi: 10.1063/1.2335683 [18] REICHLIN R, SCHIFERL D, MARTIN S, et al.Optical studies of nitrogen to 130GPa[J]. Physical Review B, 1985, 55(14):1464. [19] YAKUB L N.Polymerization in highly compressed nitrogen[J]. Low Temperature Physics, 2016, 42(1):1-16. doi: 10.1063/1.4940225 [20] GONCHAROV A F, CROWHURST J C, STRUZHKIN V V, et al.Triple point on the melting curve and polymorphism of nitrogen at high pressure[J]. Physical Review Letters, 2008, 101(9):095502. doi: 10.1103/PhysRevLett.101.095502 [21] EREMETS M I, GAVRILIUK A G, TROJAN I A.Single-crystalline polymeric nitrogen[J]. Applied Physics Letters, 2007, 90(17):171904. doi: 10.1063/1.2731679 -






 下载:
下载:




