Raman Scattering Investigation of Tetramethylsilane under High Pressure
-
摘要: 选用四甲基硅烷作为富氢电子材料,利用拉曼光谱,分析其在室温高压(68.9~142.2 GPa)下的振动模式和结构特性。结果表明:随着压力的增大,四甲基硅烷仅保留常压下的3个振动模式,且均被压力锁定;从72.2 GPa开始,出现了新的振动模式,且均随着压力的增大而发生软化,预示着四甲基硅烷可能即将半金属化。Abstract: The vibrational and structural properties of tetramethylsilane were investigated using the Raman scattering measurements under pressures ranging from 68.9 to 142.2 GPa and at room temperature.The results revealed that 3 vibrational modes of tetramethylsilane under 68.9 GPa remain locked in a certain position and are quite stable upon compression to 142.2 GPa.Moreover, new vibrational modes appear with the pressure going up to 72.2 GPa and all exhibit softening with further compression, suggesting that tetramethylsilane will become semi-metallic under such high pressures.
-
Key words:
- high pressure /
- tetramethylsilane /
- Raman spectroscopy /
- vibrational mode /
- semi-metallic
-
1. 引言
作为现代物理学中最具挑战性的课题之一,氢金属化的实现一直以来都是高压科技发展的源动力[1]。这是因为在足够高的压力(大于400 GPa)作用下,氢不仅会经历金属化过程,还可能是具有室温级超导转变温度的超导体[2-3]。百年超导发展历史中,高压下氢的研究丰富多彩。最近的实验研究[4-5]表明,加压至360 GPa时尚未发现氢实现金属化,仅呈半金属状态,而这一压力是目前静高压实验技术的上限。2004年Ashcroft[6]提出:由于其他元素对氢的“化学预压”作用,选择一些氢丰富的材料(统称富氢电子材料)代替氢,在现有的高压技术条件下将使氢金属化变得更易于实现。该方法很快被采纳,各种富氢电子材料不断涌现,其中第四主族元素的氢化物最受推崇。有研究表明,硅烷(SiH4)在60 GPa的压力下实现了金属化,在96~120 GPa获得17.5 K的超导转变温度[7-8];但是最近的实验研究却提出质疑,并证实该“金属化”源于高压下硅烷的分解产物与金属封垫化合而成的金属氢化物,而超导现象则源于硅烷的分解产物——氢在高压下与电极材料的化合产物PtH[9-11]。其他的XH4(X=C, Ge, Sn)或有同样的高压分解现象,或因剧毒较难开展高压研究。为此,富氢材料XH4(X=C, Si, Ge, Sn)失去了高压下氢金属化探索的优越性,选择合适的富氢电子材料成为研究高压下氢金属化的关键。
由于第四主族元素四甲基类材料的分子结构具有高对称性,因此自20世纪30年代其光谱特性就已被广泛研究[12-17]。就结构而言,第四主族元素四甲基类材料是将XH4中的氢基用甲基基团取代,因此二者具有相同的对称性,即Td点群对称。低温条件下该系列材料的研究[13, 18-21]表明,甲基基团在材料结构变化中起着至关重要的作用。相比于低温条件,高压环境下材料的相变行为更为丰富,展示出许多新结构和新现象。然而,对于第四主族元素四甲基类材料,与之相关的高压研究却鲜有报道。作为典型的第四主族元素四甲基类材料,四甲基硅烷(Tetramethylsilane,TMS)一般用作航空燃料和核磁共振定标物质。前期的研究[22]表明,高压下TMS有着丰富的相变,0.6 GPa时就由液态变为单晶体。考虑到拉曼光谱对相变的反映非常敏锐,是探索高压下材料相变最为直接的手段之一,因此本研究利用拉曼光谱分析TMS在较高压力范围(68.9~142.2 GPa)内的相变过程,探索TMS金属化的可能性。
2. 实验
在高压拉曼光谱实验中,高压的产生由Piston-Cylinder型金刚石对顶砧(Diamond Anvil Cell,DAC)实现,金刚石砧面直径为100 μm,带有倒角。采用钨片作为封压垫片,提前预压至20 GPa。压痕中心采用激光打孔,孔径约30 μm,作为样品腔。压力校准采用金刚石定标。由于是液态样品,实验中未使用任何传压物质。采用功率为25 mW的氦氖激光器激发拉曼光谱,探测器配套光栅的分辨率选取900 mm-1,光谱精度为1 cm-1。实验前,采用氖线标定拉曼波数,不确定度为±1 cm-1。
3. 结果与讨论
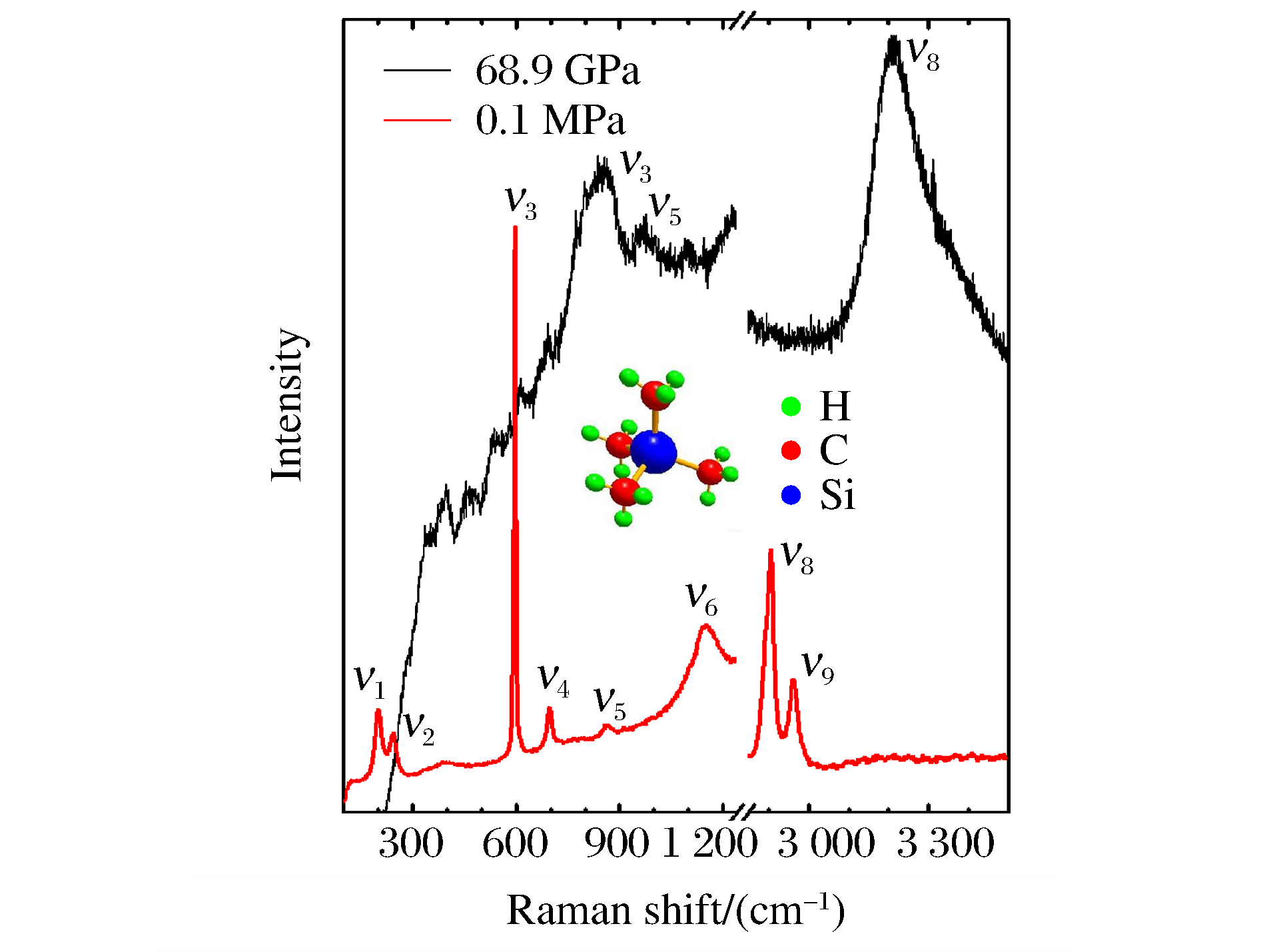
TMS的分子式为Si(CH3)4,分子结构如图 1中的插图所示:在单个TMS分子内,Si原子连接4个甲基基团,甲基基团与Si─C键完全交错,有序地排列在Si原子的周围。对于理想的四角Td(43m)分子对称,用C─H键和Si─C键的键长以及Si─C─H键或C─Si─C键的键角就可以描述整个分子的几何位置[23],其不可约表示为Γ=3A1+A2+4E+4F1+7F2[24]。根据选择定则,A1、E、F2 3种振动模式具有拉曼活性,如果不考虑振动模式的简并,共有14个拉曼振动模式(见表 1)。图 1中红色谱线为常压下测得的TMS的拉曼光谱,由于金刚石在1 332 cm-1处的强荧光影响,本研究没有标记振动模式ν7;其他振动模式和振动类别均取得了很好的匹配(见表 1)。随着压力增大到68.9 GPa,只有ν3、ν5和ν8 3个振动模式(峰位列于表 1)继续出现,其他模式均已无法识别,如图 1中黑色谱线所示。低频区(300~700 cm-1)出现的连续拉曼信号则是由样品腔前、后表面的光反射叠加而形成,而非样品的新振动模式。
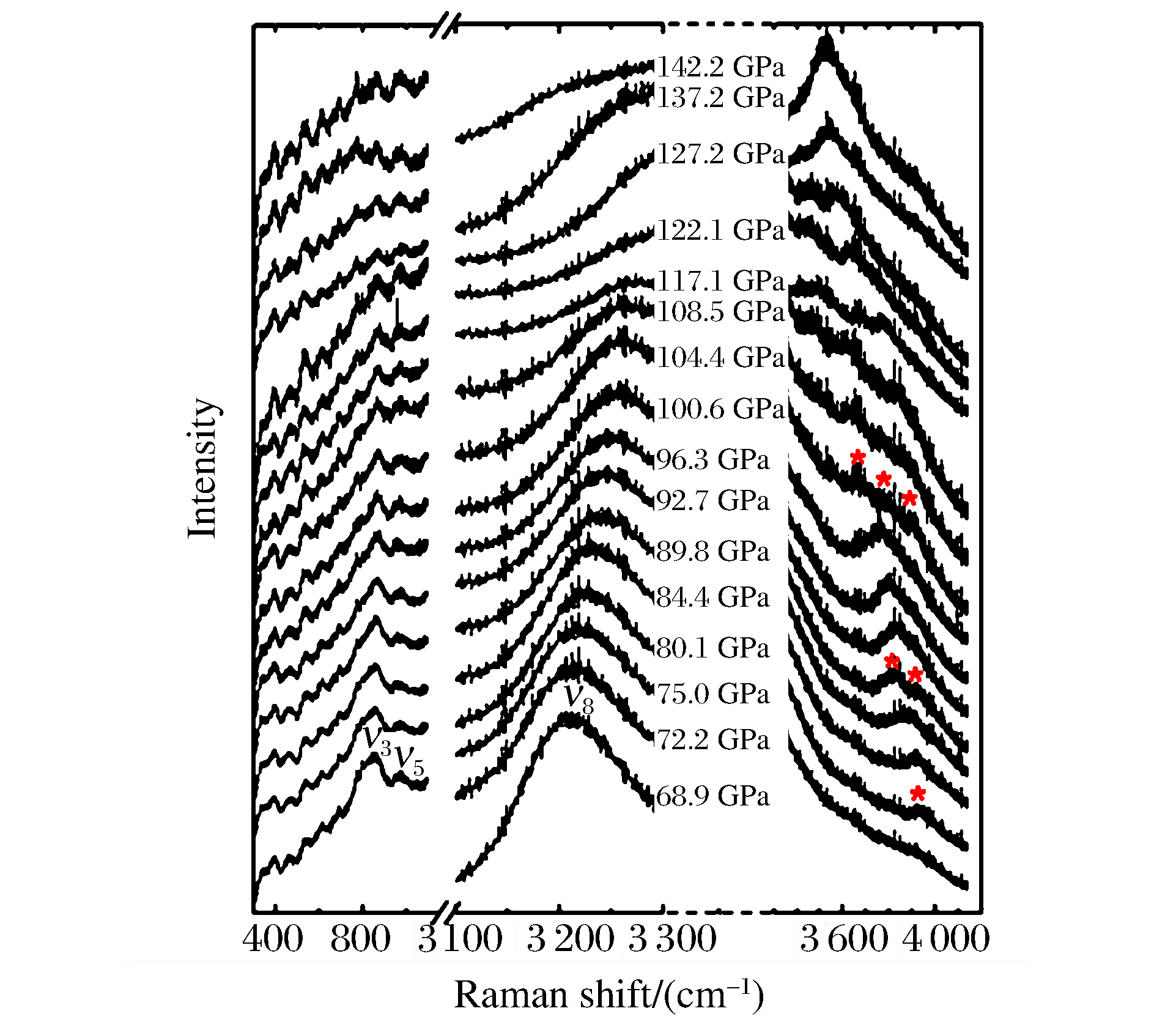
表 1 常压和高压下观测的TMS拉曼振动模式的类别和峰位Table 1. Raman shifts and assignments of the observed vibrational modes of TMS under ambient and high pressuresVibrational mode Vibrational type Raman shift/(cm-1) 0.1 MPa 68.9 GPa 72.2 GPa 84.4 GPa 100.6 GPa 122.1 GPa ν1 E(C─Si─C skeletal deformation) 201 ν2 F2(C─Si─C skeletal deformation) 244 ν3 A1(C─Si skeletal stretch) 595 848 853 865 872 869 ν4 F2(C─Si skeletal stretch) 697 ν5 E(CH3 rocking), F2(CH3 rocking) 869 968 976 971 970 983 ν6 A1(CH3 symmetrical deformation), F2(CH3 symmetrical deformation) 1 265 ν7 E(CH3 nonsymmetrical deformation), F2(CH3 nonsymmetrical deformation) 1 420 ν8 E(CH3 symmetrical stretch), F2(CH3 symmetrical stretch) 2 903 3 214 3 218 3 236 3 259 3 296 ν9 E(CH3 nonsymmetrical stretch), F2(CH3 nonsymmetrical stretch) 2 963 ν10 3 649 3 465 ν11 3 924 3 827 3 801 3 634 ν12 3 922 3 903 图 2显示了TMS在68.9~142.2 GPa压力区间的拉曼光谱变化情况。可以看出,68.9 GPa压力下仅能观察到3个振动模式,即ν3、ν5和ν8。随着压力的增大,低频区的振动模式ν3和ν5保持稳定,二者的相对强度趋于一致,且整体减弱,并慢慢淹没在样品腔前、后面的光反射叠加而形成的一系列连续拉曼信号中;振动模式ν8也有着同样的情况。在高频区出现了新奇的现象:在72.2 GPa压力下,一个新的振动模式(ν11)出现,且随着压力的增大而发生红移,即发生模式“软化”[25-27];当压力达到84.4和100.6 GPa时,分别在3 922和3 649 cm-1处出现两个新的振动模式(分别为ν12和ν10),并且两个新模式均随着压力的增大而发生软化;在117.1 GPa压力下,新模式ν12无法辨别,其原因在于该振动模式在红移过程中淹没在低频区的拉曼信号中;当压力达到137.2 GPa时,ν8与新模式发生叠加,很难分辨3个原有振动模式的峰位。表 1列出了不同压力下新模式的峰位。分析高压下TMS的拉曼光谱后发现:原有的3个模式(ν3、ν5和ν8)保持稳定,说明压力进一步限制了分子中原子间的相互作用,使分子内原有的振动模式近乎“冻结”;随着压力的增大,新模式的软化行为预示着材料的分子间或分子内的原子间出现了新的振动状态,分子间或分子内部有新的成键和组合方式,TMS在本实验的压力区间内发生了结构相变。
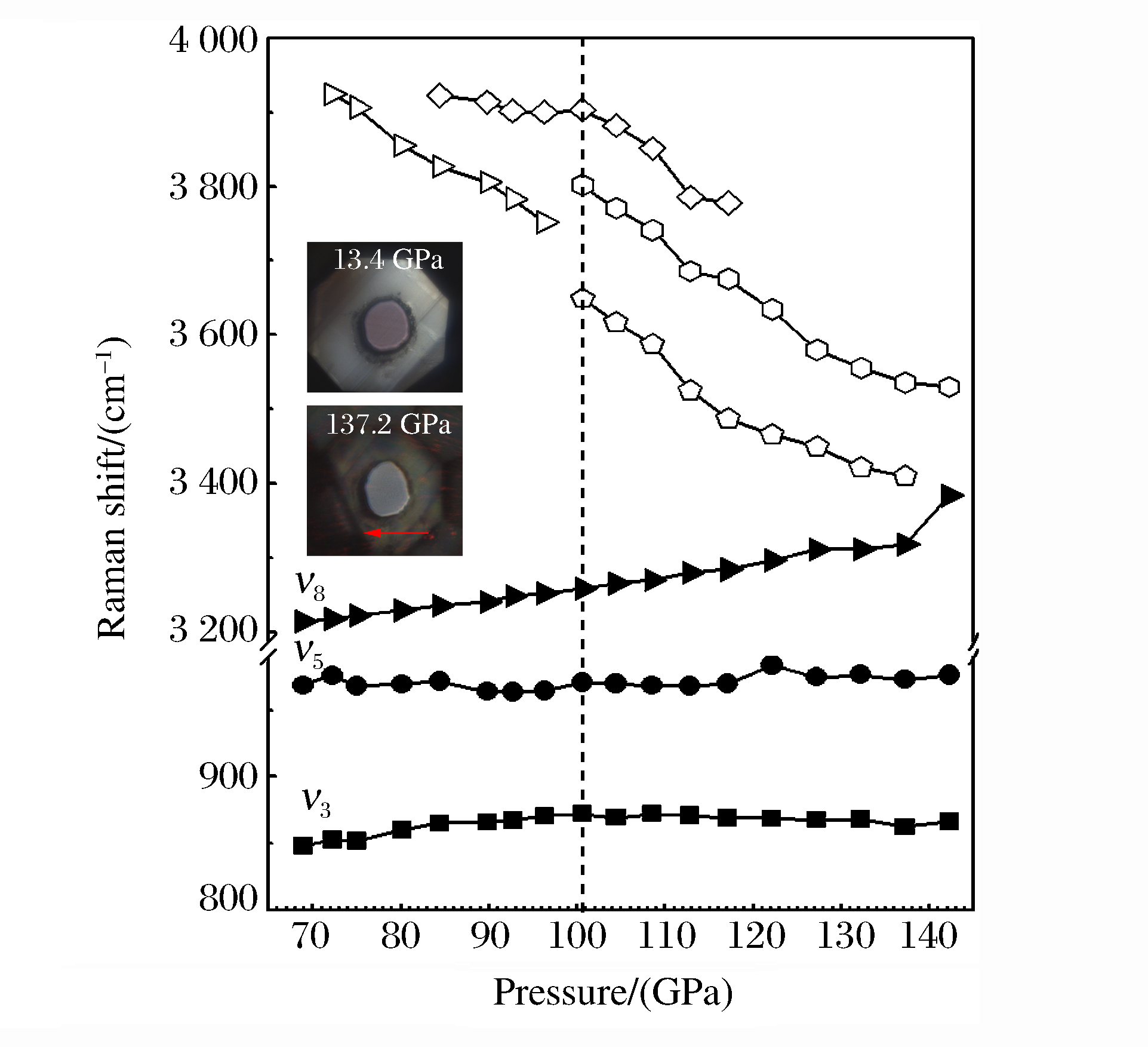
为了显示可能出现的高压相变,将拉曼振动模式的频移随压力变化的关系绘制于图 3,包括原有模式(ν3、ν5和ν8)和新出现的模式。从图 3中可以看出,模式ν3和ν5的拉曼频移-压力曲线近似为直线。在本实验压力范围内,C─Si键的拉伸模式和甲基基团的摆动模式被压力限制,只在某个特定位置上振动[28],此时TMS的分子振动与硅烷(SiH4)相似。模式ν8有着比较可观的蓝移速率,说明在本实验的压力区间内压力的作用进一步缩小了甲基基团的对称拉伸振动范围。对于新出现的振动模式,可以清楚地看到,它们有着更为明显的软化速率;100.6 GPa压力下出现的新模式劈裂(图 3中虚线所示位置)显示出分子间或分子内的复杂变化,这必然伴随着相变的发生。该现象在分子氢的高压实验中也有发现[4]:加压至220 GPa时,在原本轻微软化的拉曼振动模式附近出现了多个新模式,并且这些新模式随着压力的增加也发生软化。氢的常温高压可见光吸收谱实验证实,在278 GPa压力下氢可能处于半金属状态[4]。另外,该现象在最近报道的硫化氢(H2S)高压实验[29]中也被观测到,即伴随着部分拉曼振动模式软化行为的发生,在约90 GPa时硫化氢实现了金属化,高压低温电输运测量得到其超导转变温度为190 K。上述研究表明,高压下分子间或分子内基团振动模式所发生的软化行为不仅是结构相变的有力证据,而且也是高压下富氢材料实现金属化的预兆和特征,使得TMS在高压作用下能够实现金属化的推断更为合理。图 3中的插图显示了加压至137.2 GPa时TMS样品情况,对比13.4 GPa压力下的样品形态,可以看出TMS样品仍是透明的,说明此时TMS尚未金属化。当压力达到137.2 GPa时,金刚石台面发生断裂(图 3插图中箭头所指处),无法继续加压。考虑到TMS在72.2 GPa时就出现振动模式的软化行为,对比高压下氢的实验结果,显而易见,相对于220 GPa的压力,72.2 GPa更易获得,大大提高了应用现有的静高压实验技术实现TMS金属化的可能性。
 图 3 TMS拉曼振动模式的频移与压力的关系(实心符号表示原有模式,空心符号表示新模式,100.6 GPa处的竖直虚线表示相界,插图显示显微镜观测的样品腔形态)Figure 3. Raman shifts vs. pressure for the observed modes of TMS (The solid and hollow symbols represent original and new modes respectively, and the vertical dashed line under 100.6 GPa indicates the proposed phase boundary.The insets show the stressed samples via a microscope)
图 3 TMS拉曼振动模式的频移与压力的关系(实心符号表示原有模式,空心符号表示新模式,100.6 GPa处的竖直虚线表示相界,插图显示显微镜观测的样品腔形态)Figure 3. Raman shifts vs. pressure for the observed modes of TMS (The solid and hollow symbols represent original and new modes respectively, and the vertical dashed line under 100.6 GPa indicates the proposed phase boundary.The insets show the stressed samples via a microscope)4. 结论
利用拉曼光谱学,进行了高压下TMS金属化行为的研究。在68.9~142.2 GPa压力范围内,仅存的3个原有振动模式进一步被压力限制,其中甲基基团对称拉伸模式的振动空间缩小,C─Si键的拉伸模式和甲基基团的摆动模式在低于68.9 GPa压力下就已被锁定。当压力达到72.2和84.4 GPa时,观察到两个新模式,并且随着压力的升高,新模式表现出软化行为;在100.6 GPa压力下,伴随着拉曼峰的劈裂,又出现了新的振动模式,并且同样随着压力的增加而发生软化。这一奇特的分子间或分子内基团振动现象说明:伴随着相变的发生,TMS可能即将进入半金属态。由于所需压力较易获得,因此利用目前的静高压实验技术实现此类富氢电子材料的高压金属化是非常可行的。
-
图 3 TMS拉曼振动模式的频移与压力的关系(实心符号表示原有模式,空心符号表示新模式,100.6 GPa处的竖直虚线表示相界,插图显示显微镜观测的样品腔形态)
Figure 3. Raman shifts vs. pressure for the observed modes of TMS (The solid and hollow symbols represent original and new modes respectively, and the vertical dashed line under 100.6 GPa indicates the proposed phase boundary.The insets show the stressed samples via a microscope)
表 1 常压和高压下观测的TMS拉曼振动模式的类别和峰位
Table 1. Raman shifts and assignments of the observed vibrational modes of TMS under ambient and high pressures
Vibrational mode Vibrational type Raman shift/(cm-1) 0.1 MPa 68.9 GPa 72.2 GPa 84.4 GPa 100.6 GPa 122.1 GPa ν1 E(C─Si─C skeletal deformation) 201 ν2 F2(C─Si─C skeletal deformation) 244 ν3 A1(C─Si skeletal stretch) 595 848 853 865 872 869 ν4 F2(C─Si skeletal stretch) 697 ν5 E(CH3 rocking), F2(CH3 rocking) 869 968 976 971 970 983 ν6 A1(CH3 symmetrical deformation), F2(CH3 symmetrical deformation) 1 265 ν7 E(CH3 nonsymmetrical deformation), F2(CH3 nonsymmetrical deformation) 1 420 ν8 E(CH3 symmetrical stretch), F2(CH3 symmetrical stretch) 2 903 3 214 3 218 3 236 3 259 3 296 ν9 E(CH3 nonsymmetrical stretch), F2(CH3 nonsymmetrical stretch) 2 963 ν10 3 649 3 465 ν11 3 924 3 827 3 801 3 634 ν12 3 922 3 903 -
[1] GINZBURG V L.Nobel lecture:on superconductivity and superfluidity (what I have and have not managed to do) as well as on the "physical minimum" at the beginning of the 21st century[J].Rev Mod Phys, 2004, 76(3):981-998. doi: 10.1103/RevModPhys.76.981 [2] ASHCROFT N W.Metallic hydrogen:a high-temperature superconductor?[J].Phys Rev Lett, 1968, 21(26):1748-1749. doi: 10.1103/PhysRevLett.21.1748 [3] RICHARDSON C F, ASHCROFT N W.High temperature superconductivity in metallic hydrogen:electron-electron enhancements[J].Phys Rev Lett, 1997, 78(1):118-121. http://adsabs.harvard.edu/abs/1997PhRvL..78..118R [4] HOWIE R T, GUILLAUME C L, SCHELER T, et al.Mixed molecular and atomic phase of dense hydrogen[J].Phys Rev Lett, 2012, 108(12):125501. doi: 10.1103/PhysRevLett.108.125501 [5] ZHA C S, LIU Z, HEMLEY R J.Synchrotron infrared measurements of dense hydrogen to 360 GPa[J].Phys Rev Lett, 2012, 108(14):146402. doi: 10.1103/PhysRevLett.108.146402 [6] ASHCROFT N W.Hydrogen dominant metallic alloys:high temperature superconductors?[J].Phys Rev Lett, 2004, 92(18):187002. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0212330369/ [7] CHEN X J, STRUZHKIN V V, SONG Y, et al.Pressure-induced metallization of silane[J].Proc Natl Acad Sci, 2008, 105(1):20-23. doi: 10.1073/pnas.0710473105 [8] EREMETS M I, TROJAN I A, MEDVEDEV S A, et al.Superconductivity in hydrogen dominant materials:silane[J].Science, 2008, 319(5869):1506-1509. doi: 10.1126/science.1153282 [9] HANFLAND M, PROCTOR J E, GUILLAUME C L, et al.High-pressure synthesis, amorphization, and decomposition of silane[J].Phys Rev Lett, 2011, 106(9):095503. doi: 10.1103/PhysRevLett.106.095503 [10] STROBEL T A, GONCHAROV A F, SEAGLE C T, et al.High-pressure study of silane to 150 GPa[J].Phys Rev B, 2011, 83(14):144102. doi: 10.1103/PhysRevB.83.144102 [11] SCHELER T, DEGTYAREVA O, MARQUES M, et al.Synthesis and properties of platinum hydride[J].Phys Rev B, 2011, 83(21):214106. doi: 10.1103/PhysRevB.83.214106 [12] PRATT L R, HSU C S, CHANDLER D.Statistical mechanics of small chain molecules in liquids. Ⅰ. effects of liquid packing on conformational structures[J].J Chem Phys, 1978, 68(9):4202-4213. doi: 10.1063/1.436284 [13] SILVER S.The normal vibrations of molecules of the tetramethylmethane type[J].J Chem Phys, 1940, 8(12):919-933. doi: 10.1063/1.1750606 [14] WATARI F.Vibrational spectra of (CD3)4M, and normal coordinate calculations for (CH3)4M and (CD3)4M (M=Si, Ge, Sn, Pb)[J].Spectrochim Acta A, 1978, 34(12):1239-1244. doi: 10.1016/0584-8539(78)80087-7 [15] RANK D H.The Raman spectrum of tetramethylmethane[J].J Chem Phys, 1933, 1(8):572-575. doi: 10.1063/1.1749330 [16] EDSALL J T.Raman spectra of amino acids and related substances Ⅲ.ionization and methylation of the amino group[J].J Chem Phys, 1937, 5(4):225-237. doi: 10.1063/1.1750013 [17] SHELINE R K, PITZER K S.The infra-red spectrum of tetramethyl lead and the force constants of M(CH3)4 type molecules[J].J Chem Phys, 1950, 18(5):595-601. doi: 10.1063/1.1747707 [18] PERRY S, JONAS J.High pressure Raman study of vibrational relaxation in liquids of group Ⅳ tetramethyl compounds[J].J Chem Phys, 1983, 79(12):6308-6311. doi: 10.1063/1.445738 [19] MONES A H, POST B.X-ray diffraction study of crystalline neopentane (tetramethyl methane)[J].J Chem Phys, 1952, 20(4):755-756. doi: 10.1063/1.1700547 [20] VALERGA A J, KILPATRICK J E.Entropy and related thermodynamic properties of tetramethylgermane[J].J Chem Phys, 1970, 52(9):4545-4549. doi: 10.1063/1.1673681 [21] KREBS B, HENKEL G, DARTMANN M.Structure of tetramethyltin, Sn(CH3)4[J].Acta Cryst C, 1989, 45:1010-1012. doi: 10.1107/S0108270189000090 [22] QIN Z X, ZHANG J B, TROYAN I, et al.High-pressure study of tetramethylsilane by Raman spectroscopy[J].J Chem Phys, 2012, 136(2):024503. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=71977d5b7ef88c956b468178879830b8 [23] GAJDA R, KATRUSIAK A.The interplay of molecular conformation and crystal packing in pressure-frozen tetramethylsilane[J].Cryst Growth Des, 2008, 8(1):211-214. doi: 10.1021/cg070135h [24] SHIMIZU K, MURATA H.Normal vibrations and calculated thermodynamic properties of tetramethylsilane[J].J Mol Spectrosc, 1960, 5:44-51. http://www.sciencedirect.com/science/article/pii/0022285261900649 [25] ADAMS D M, POGSON M.Vibrational spectroscopy at very high pressures.part 48.a Raman study of the phase behaviour of CH3HgX (X=Cl, Br, I), and the crystal structure of CH3HgBr[J].J Phys C, 1988, 21(6):1065-1079. doi: 10.1088/0022-3719/21/6/013 [26] RUSH J J, HAMILTON W C.Free rotation of methyl groups in dimethyltin difluoride[J].Inorg Chem, 1966, 5(12):2238-2239. doi: 10.1021/ic50046a037 [27] ADAMS D M, HAINES J.Phase transitions in the dimethyl thallium (Ⅲ) halides, (CH3)2TlX (X=Cl, Br, I)[J].Spectrochim Acta A, 1993, 49(2):237-248. http://www.sciencedirect.com/science/article/pii/058485399380178D [28] LIN Y, MAO W L, DROZD V, et al.Raman spectroscopy study of ammonia borane at high pressure[J].J Chem Phys, 2008, 129(23):234509. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_PM19102540 [29] DROZDOV A P, EREMETS M I, TROYAN I A.Conventional superconductivity at 190 K at high pressures[EB/OL].[2015-09-10].http://arxiv.org/abs/1412.0460. -








 下载:
下载:


 下载:
下载: