Effect of High Pressure-Enzymolysis on Solubility and Structural Characteristic of Rice Protein
doi: 10.11858/gywlxb.2014.06.015
-
Abstract: Thermo denatured rice protein was treated by 100 MPa for 10 min at 25 ℃ and then was hydrolyzed with Alcalase.The solubility and structural characterization of the protein and its hydrolysates were analyzed by size exclusion-high performance liquid chromatography (SE-HPLC), Fourier transform infrared spectroscopy (FTIR) and scanning electric microscope (SEM) so as to evaluate the effect of high pressure.The results showed that the solubility of hydrolyzed rice protein after high pressure treated could elevate to 75.33% from 58.9% of non-high pressure treated.The 57-105 ku fraction of the protein showed a dissolved state after high pressure treatment, and the contents of 4.4 and 2.0 ku fractions increased during the hydrolysis of the protein, which were different from that of non-high pressure treated.The β-sheet and β-turn were the major conformation of the protein.The structure of the protein body particles became loosen and easier to be hydrolyzed after high pressure treatment.These results show that 100 MPa treatment could promote the enzymatic hydrolysis of thermo-denatured rice protein.
-
Key words:
- rice protein /
- high-pressure /
- enzymatic hydrolysis /
- structural characterization /
- FTIR
摘要: 研究了100 MPa高压和酶解处理对热变性大米蛋白溶解性和分子结构特征的影响。结果显示:高压处理后再用Alcalase(碱性蛋白酶)酶解可使大米蛋白的溶解性由单纯酶解时的58.9%提高到75.33%;SE-HPLC(高效液相凝胶色谱法)分析显示, 高压处理可使57~105 ku的大分子蛋白质溶出, 且随着酶解时间延长, 该组分消失, 4.4和2.0 ku组分含量增加, 而非高压处理者没有大分子的溶解; FTIR(傅里叶红外光谱)分析显示, 高压处理的样品中β-折叠和β-转角结构占主导地位; SEM(扫描电子显微镜)分析表明, 高压处理使致密的大米蛋白体结构变得疏松。以上结果表明, 高压处理改变了蛋白的空间结构, 进而改变蛋白的酶解位点, 从而提高了大米蛋白的溶解性。 -
1. Introduction
Rice is the main grain in China and some other countries in the world, it is not only an important staple food for millions of people, but also a significant material for many industries such as syrup, organic acids and antibiotics fermentation.At the same time, rice protein, which has a reasonable amino acid composition and hypoallergenic properties, is considered as a high-quality plant protein that may be useful in infant formulations[1-2], and its important health care function, such as application in hyperlipidemia, cholesterol-lowering, anti-leukaemia, antihypertensive, has also been reported[3-5].
Thermo-denatured rice protein (TDRP) powder containing 50% protein was a by-product from the process of rice syrup production.The by-product yields are about 1/7 of the rice material used in those production processes, which suggests a significant source of plant protein.But the rice protein has been heated at above 92 ℃ in the process and results in serious thermal denaturation, lots of conventional technologies and methods on solubility and structural characterization of protein cannot perform effectively, and only a few related researches have been reported.Therefore, it is of significance to find a way to efficiently utilize this protein source.
Many researches have shown that high-pressure (HP) treatment can influence the three-dimensional structure of protein, and bring about reversible or irreversible changes of protein conformation.The degree of the changes induced by HP depends not only upon the level and duration time of pressure, but also the type of protein, pH, and temperature[6].The enzymatic hydrolysis of HP treated protein has different phenomenon from that of ordinary protein due to the change of protein structure.For example, β-lactoglobulin (β-Lg) of milk is not easy to be hydrolyzed by pepsin at atmospheric pressure, but it is easily enzymatic hydrolyzed at 200 MPa, and there is no whole β-Lg molecular existing in the solution hydrolyzed with pepsin for 10 min at 400 MPa[7-8].Most of the peptide products of HP treated (600 MPa) β-Lg hydrolyzed by pepsin for 1 min were less than 1 500 u in size[9].
This study aimed to investigate the changes in the solubility, molecular weight distribution and conformation of rice protein during its enzymolysis after 100 MPa HP treatment.
2. Materials and Methods
2.1 Materials
The thermo-denatured rice protein (TDRP) was donated by Hanke Bioengineering Ltd., China, it was a by-product from the process of rice syrup production.Protein content of the sample was determined by Kjeldahl method as 61.98%.Alcalase 2.4L (2.4 AU/g) were purchased from Novozymes, Denmark.Molecular weight (MW) markers were rabbit phosphorylase b (97.4 ku), bovine serum albumin (66.2 ku), rabbit actin (43 ku), carbonic bovine carbonic anhydrase (31 ku), trypsin inhibitor (20.1 ku), and hen egg white lysozyme (14.4 ku).All other chemicals were of analytical grade.
2.2 High-Pressure Processing
The TDRP was suspended as a 5% (mass concentration) solution in 30 mL distilled water adjusted to pH 8.0 with 1 mol/L NaOH.The solutions were conditioned vacuum in a double polyethylene bag and subjected to 100 MPa high-pressure treatment for 10 min.The high-pressure processing of rice protein samples was carried out in a 5 L vessel (UHP900×2-Z, Wentian Ltd., Baotou, China) equipped with temperature and pressure regulator device, and oil was used as a fluid of low compressibility.Temperature of medium in the vessel was settled at 25 ℃.The loading speed was 10 MPa/s, and after hold at 100 MPa for 10 min, the pressure was quickly released (300 MPa/s) to atmospheric pressure.Each sample was replicated 3 times.And then, the HP treated substrate was utilized for proteolysis at atmospheric pressure.
2.3 Enzymatic Hydrolysis of Rice Proteins
Hydrolysis was performed at atmospheric pressure (0.1 MPa) for 30, 60, 90, 120 min with 15 μL Alcalase solution, using HP treated and untreated (control) substrate, respectively.The temperature of reaction was 50 ℃.The pH was kept at 8.0 by adding 1 mol/L NaOH, according to pH-stat technique[10].The same reactions were performed without any enzyme addition to prepare the blanks of the experiment.The derived hydrolysates were heated (95 ℃, 15 min) and centrifuged at 3 500 r/min for 10 min at 25 ℃.The soluble and insoluble rice protein were freeze-dried (LYOVACGT2 Vacuum Freeze-dried Instrument, SRK-SYSTEM TECHNIK Company, Germany).The crude protein content of both preparations was determined by micro-Kjeldahl method according to Petrucelli and A
 on[11].The coefficient of protein-nitrogen was 5.95.
on[11].The coefficient of protein-nitrogen was 5.95.2.4 SE-HPLC
The hydrolysates were filtered (0.45 μm) and loaded on a TSK-GEL G2000SWXL (Tosoh, Japan) steel column (7.8 mm×300 mm) connected to a LC600 System (LabTech, Beijing, China).Separation was conducted at 30 ℃ with a flow rate of 1.0 mL/min, the mobile phase was acetonitrile/water/TFA (45/55/0.1).The eluted proteins were detected at 215 nm.
2.5 FTIR Spectroscopy
Infrared spectra of insoluble rice protein were recorded by a Nicolet 5700 FTIR spectrometer (Thermo Company, USA) equipped with a DTGS detector and a Ge/KBr beamsplitter.Samples were mixed with KBr, and tablet pressed.The tablet was contained in Golden Gate Diamond ATR (Attenuated Total Teflectance) system.A total of 32 scans were averaged at 4 cm-1 resolution.Deconvolution of infrared spectra was performed using Peakfit version 4.12 (Seasolve Software Inc.) according to Byler et al.[11].The half width used for deconvolution was 10 cm-1 and the filter 60%.To ensure that the spectra were not over-deconvoluted, the acquired spectra were judged by evaluating the second derivative spectra, comparing the number and position of bands with the deconvolution spectra.Band assignment in the amide Ⅰ region (1 600-1 700 cm-1) was determined according to Ref.[11].Quantitative analysis of secondary structure components was performed using Gaussian peaks and curve fitting models.
2.6 Scanning Electron Microscopy (SEM)
The 100 MPa treated and control samples were fractured with a blade, the fragments were mounted on aluminum stubs, and gold-coated in vacuum.The surface morpha of both samples were observed with an SEM (JSM-6490LV, Japan) at an accelerating voltage of 10 kV and 10 mm working distance.
3. Results and Discussion
3.1 Protein Contents of Hydrolysates
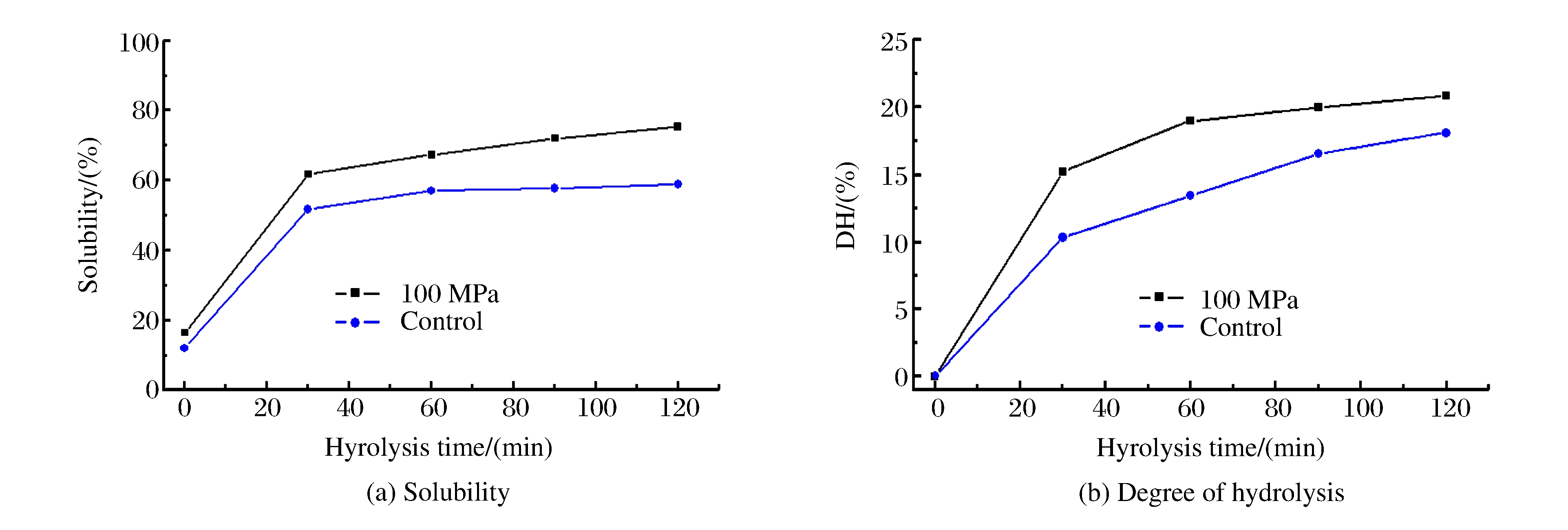
The solubility and degree of hydrolysis of rice protein were determined to evaluate the extraction efficiency under different conditions, as shown in Fig. 1.Both at control and 100 MPa, a remarkable increase of rice protein solubility was observed during the extracting time (from 0 to 30 min).Beyond that time range, there was little difference in the solubility (Fig. 1(a)), which was typical for hydrolysis curves of plant protein.The solubility values of 100 MPa treated samples varied from 16.05% to 75.33% after 120 min of hydrolysis, but that of control samples were just from 12.03% to 58.9%.Samples treated by HP gave the highest solubility value and were more efficient in rice protein hydrolysis than control samples.
But as to degree of hydrolysis (DH), it increased at a rapid rate during the initial 30 min, and went up quickly as well thereafter, especially for the control samples (Fig. 1(b)).This result did not accord with the tendency of solubility.The reason might be that soluble rice protein macromolecules degraded into small molecular-weight peptides.HP treatment changed the three-dimensional structure and the enzymatic hydrolysis feature, leading to more protein dissolving.
3.2 Gel Filtration Chromatography of Soluble Protein
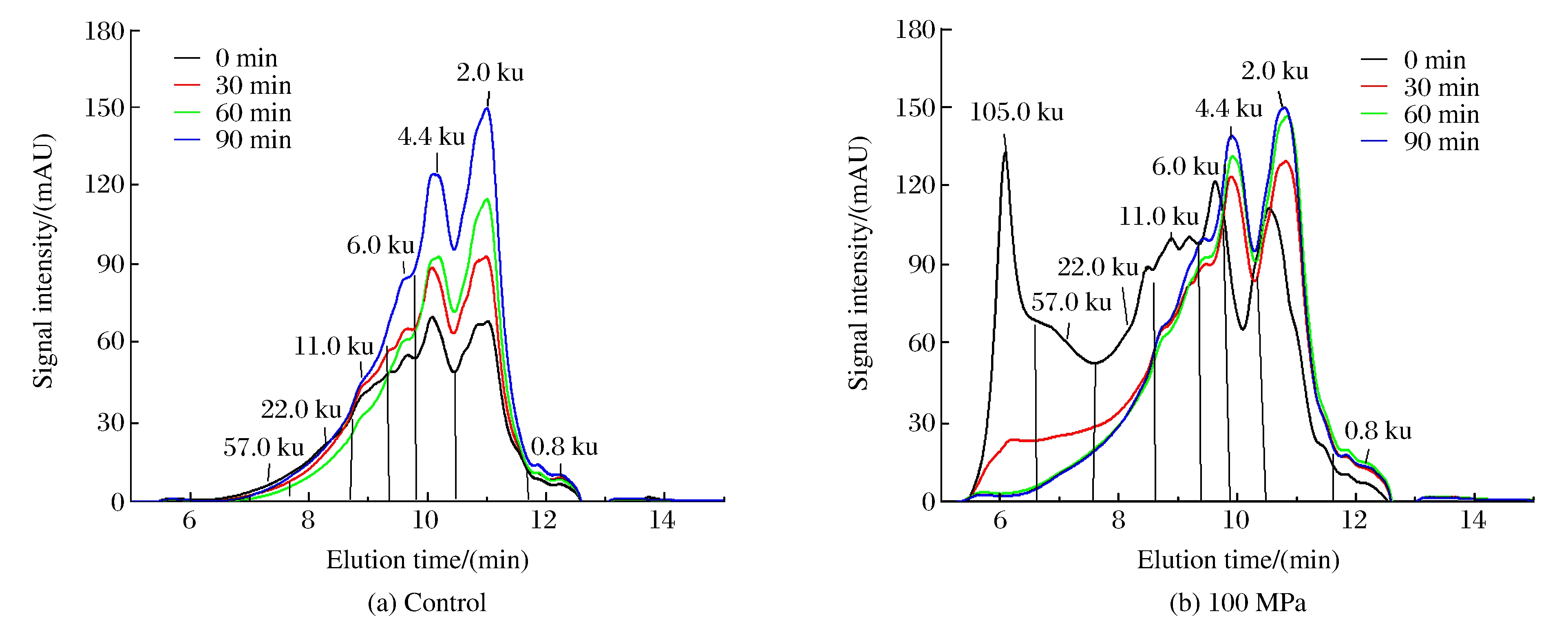
The effect of pressurization on the molecular weight distribution of rice protein was investigated by SE-HPLC, as shown in Fig. 2.Samples of control with different hydrolysis time showed similar patterns of two major peaks (fraction over 60%) whose corresponding MW were 4.4 and 2.0 ku, respectively.The fraction of 4.4 and 2.0 ku proteins increased with the hydrolysis time, which was consistent with the hydrolysis degree.At 100 MPa, the fraction of 57-105 ku proteins was 20% in the blank group, which might be caused by the cross-link between the subunits of rice protein in the process of syrup production.As the hydrolysis went along, the fraction of 57-105 ku decreased, and the fraction of 4.4 and 2.0 ku increased.Furthermore, HP treatment changed the structure of rice protein, leading to the change of enzymatic mechanism and benefit to solubility.
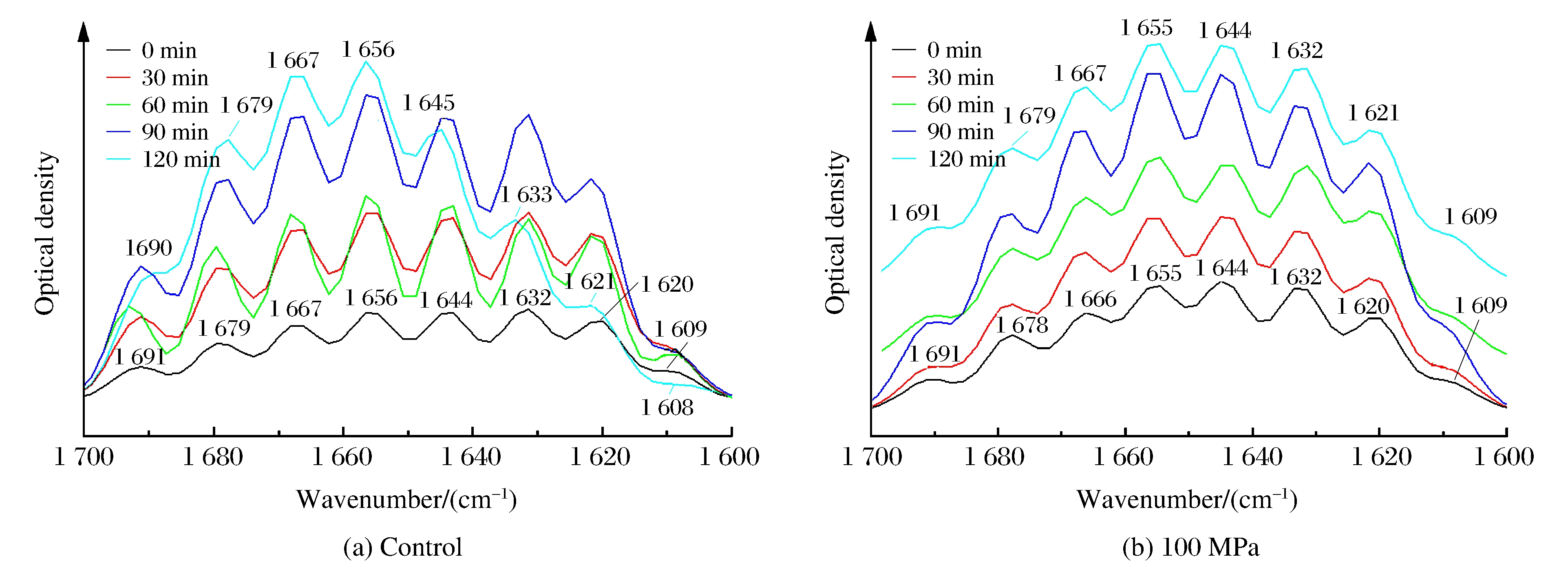
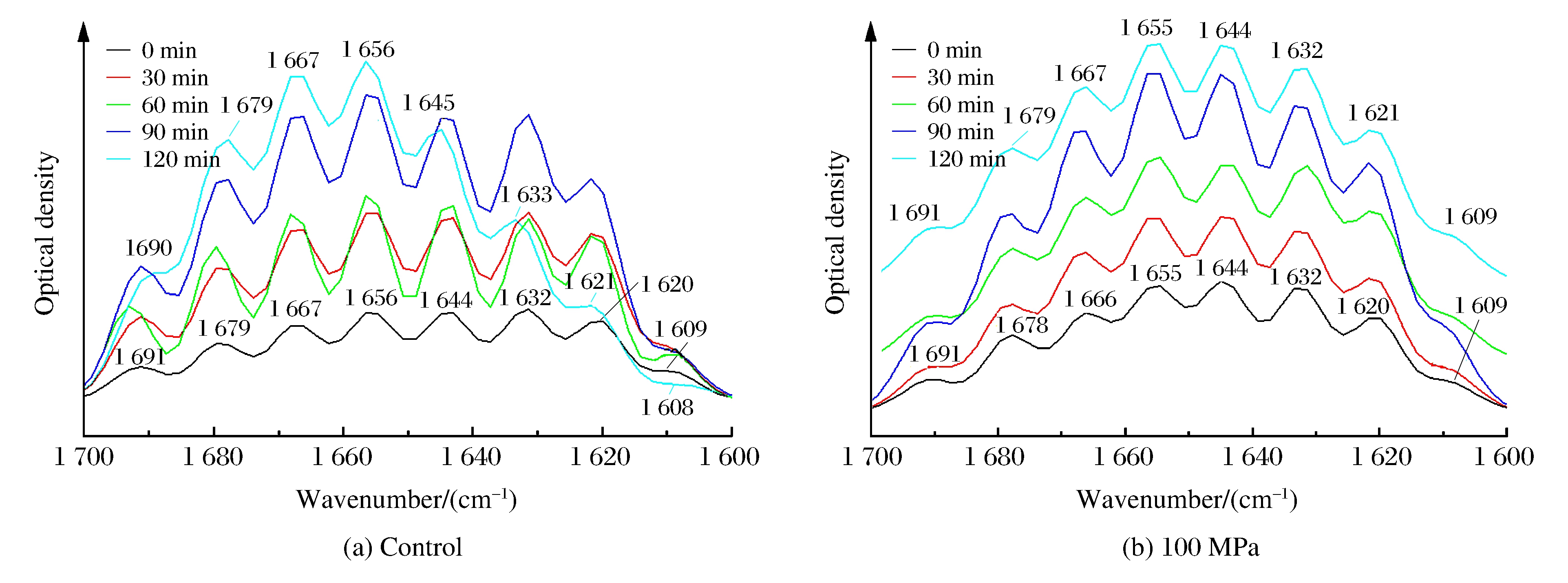
3.3 Spectral Assignment
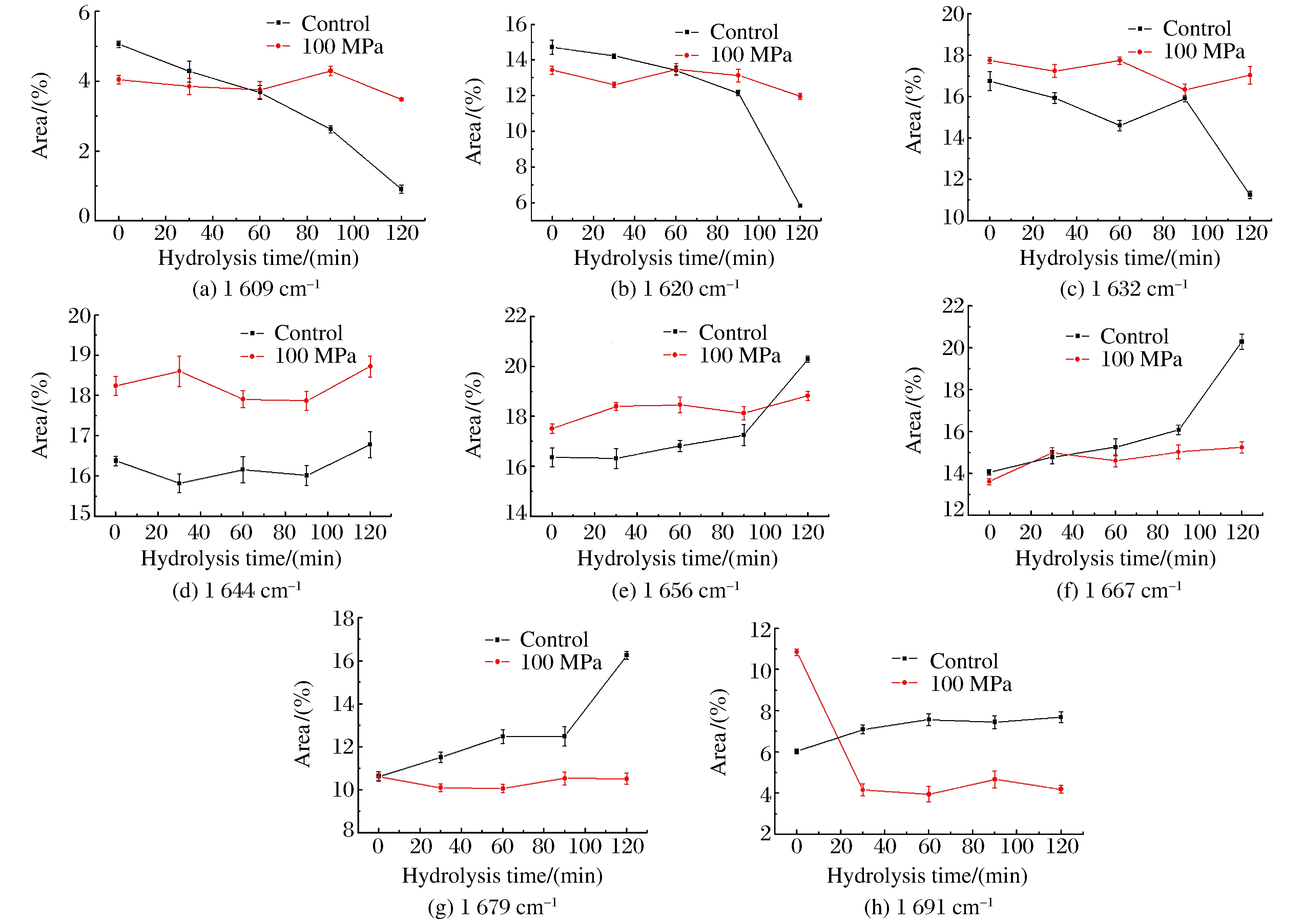
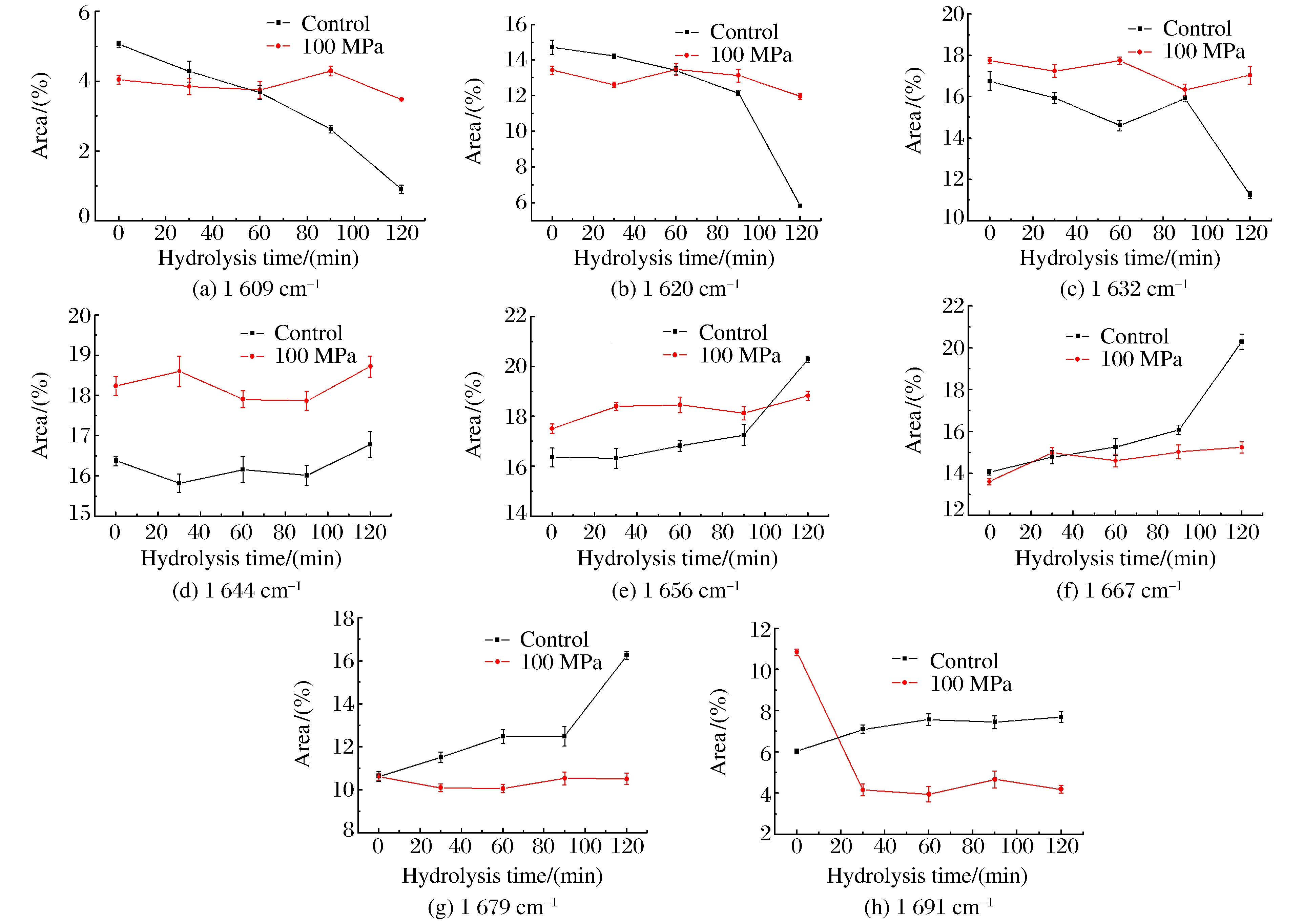
Fig. 3 shows the deconvoluted infrared spectra (1 600-1 700 cm-1) of insoluble rice protein after hydrolysis.Eight major bands were observed in the amide Ⅰ region.From the infrared spectra, the secondary structure composition of rice protein both at control and 100 MPa, estimated by integrating the major bands, was found to be predominantly β-sheets and turns (over 60%), followed by α-helices (about 20%) and random coils (about 20%), as shown in Fig. 4.A previous FTIR study showed that prolamin and glutelin exhibited α-helices and random coils as the major secondary structures[13].However, many seed globulins from monocotyledonous and dicotyledonous plants were found to possess low levels of α-helix and high levels of β-sheet secondary structure[14].Although rice protein both at control and 100 MPa exhibited similar FTIR spectral characteristics (mainly β-sheets and turns), an increase in the intensity of 1 644 and 1 691 cm-1 band and decrease in the intensity of 1 620 cm-1 suggest a transition from ordered conformation to random coils and from aggregated strands to β-sheet.The results indicate a change in hydrogen bonding or conformation after the treatment of 100 MPa.
Over time, the intensity of bands attributed to side chain vibration (1 609 cm-1), aggregated strands (1 620 cm-1) and β-strand (1 632 cm-1) decreased remarkably, while the intensity of α-helix (1 656 cm-1), β-turns (1 667 cm-1), β-sheet (1 679 cm-1) increased significantly at control.The reason might be that HP changed the granular, lamellar structure to swollen and deformed, which benefited the enzymatic hydrolysis.At the same time, we found that even β-sheets and turns were main components of rice protein structure, the solubility was still poor.The relationship between the secondary structure and solubility will be undertaken in the next phase.
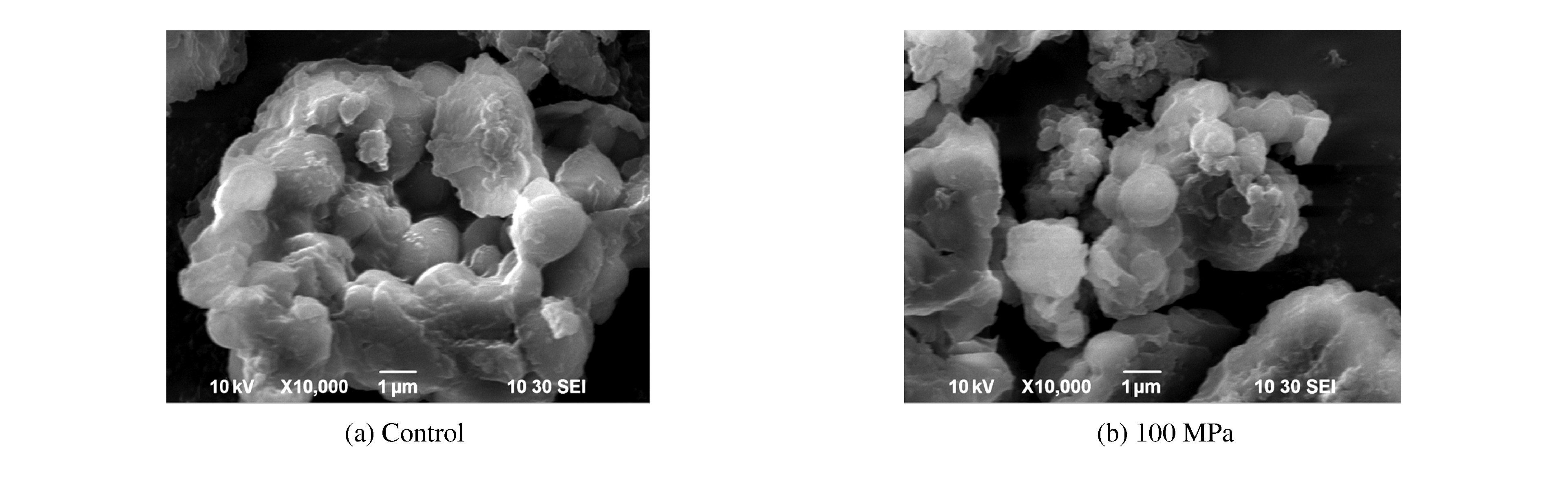
3.4 SEM Analysis
The structures of control and 100 MPa treated samples were also examined under scanning electron microscope (Fig. 5), which contributed to understand the structure of surface and analyze the molecule structure.Changes in the shape and surface of rice protein induced by 100 MPa treatment were clearly visible.At control, the rice protein presented as protein body granules and was smooth.But the protein body granule changed after treated by 100 MPa.Most granules appeared swollen and deformed.This disaggregation of protein body into small amorphous granules may be a major cause of improvement in the digestibility of 100 MPa treated rice protein.
4. Conclusions
It was confirmed in this work that enzymatic hydrolysis could significantly improve the extraction efficiency of rice protein.HP treatment showed an enhancement effect on the enzymatic hydrolysis of rice protein to some extent.Compared with control, there were 105 ku proteins existing at 100 MPa, which was different from the basic subunit of rice protein, and might be caused by aggregation.The subunits of rice protein with different processing were not markedly different.Although β-sheets and turns were the major secondary structures of rice protein, the solubility was still poor.The results indicate a change in hydrogen bonding or conformation after the treatment of 100 MPa HP, leading to the enhancement effect on the enzymatic hydrolysis.
-
-
[1] Helm R M, Burks A W. Hypoallergenicity of rice bran protein[J]. Cereal Foods World, 1996, 41(11): 839-843. [2] Juliano B O. Production and Utilization of Rice[M]. 2nd ed. Minnesota: American Association of Cereal Chemists Inc Press, 1985: 1-16. [3] Jariwalla R J. Rice-bran products: Phytonutrients with potential applications in preventive and clinical medicine[J]. Drug Exp Clin Res, 2001, 27(1): 17-26. [4] Chen Q H, Xuan G D, Fu M L, et al. Effect of angiotensin I-converting enzyme inhibitory peptide from rice dregs protein on antihypertensive activity in spontaneously hypertensive rats[J]. Asia Pac J Clin Nutr, 2007, 16: 281-285. [5] Chen Y J, Chen Y Y, Wu C T, et al. Prolamin, a rice protein, augments anti-leukaemia immune response[J]. J Cereal Sci, 2009, 51(2): 189-197. [6] Heremans K, Smeller L. Protein structure and dynamics at high pressure[J]. Biochim Biophys Acta, 1998, 1386(2): 353-370. doi: 10.1016/S0167-4838(98)00102-2 [7] Chicón R, Belloque J, Alonso E, et al. Immunoreactivity and digestibility of high-pressure-treated whey proteins[J]. Int Dairy J, 2008, 18: 367-376. doi: 10.1016/j.idairyj.2007.11.010 [8] Chicón R, Belloque J, Alonso E, et al. Antibody binding and functional properties of whey protein hydrolysates obtained under high pressure[J]. Food Hydrocolloid, 2009, 23(3): 593-599. doi: 10.1016/j.foodhyd.2008.04.001 [9] Zeece M, Huppertz T, Kelly A. Effect of high-pressure treatment on in-vitro digestibility of β-lactoglobulin[J]. Innovat Food Sci Emerg Tec, 2008, 9(1): 62-69. doi: 10.1016/j.ifset.2007.05.004 [10] van der Plancken I, van Remoortere M, van Loey A, et al. Heat-induced changes in the susceptibility of egg white proteins to enzymatic hydrolysis: A kinetic study[J]. J Agric Food Chem, 2003, 51(13): 3819-3823. doi: 10.1021/jf026019y [11] Petrucelli S, Aon M C. Relationship between the method of obtention and the structural and functional properties of soy protein isolates. 1. Structural and hydration properties[J]. J Agric Food Chem, 1994, 42(10): 2161-2169. doi: 10.1021/jf00046a017 [12] Byler D M, Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra[J]. Bioplymers, 1986, 25(3): 469-487. doi: 10.1002/bip.360250307 [13] Mawal Y R, Mawal M R, Sainani M. N, et al. Rice seed storage proteins: a structural insight[J]. Plant Sci, 1990, 70(1): 73-80. [14] Marcone M F, Kakuda Yukio, Yada R Y. Salt-soluble seed globulins of dicotyledonous and monocotyledonous plants Ⅱ. Structural characterization[J]. Food Chem, 1998, 63(2): 265-274. doi: 10.1016/S0308-8146(97)00159-3 -








 DownLoad:
DownLoad:





 下载:
下载: