Shock-Induced Desulfurization of Natural Pyrite and Its Implications for the Early Earth’s Environment
-
摘要: 陨石撞击事件被认为是早期地球大气成分变化的重要驱动因素之一,对生物演化产生了深远影响。含硫矿物在这类自然撞击事件中的物理化学行为对于理解硫元素在地球海洋-大气系统演化中的作用至关重要。为此,通过天然含硫矿物黄铁矿(FeS2)在约20和55 GPa冲击压力下的回收实验探究其在高速撞击条件下的分解特性。实验结果表明,在约55 GPa的冲击压力下,黄铁矿部分分解为磁黄铁矿和单质硫,揭示了黄铁矿在陨石撞击相关环境下具有热力学不稳定性,并伴随着硫的释放。这一脱硫反应可能为含硫气体注入海洋和大气系统提供了新的路径,由此引发的环境变化可能与约2.5亿年前二叠纪末期的生物灭绝事件存在关联,为理解该时期的生物危机提供了重要线索。Abstract: Meteorite impact events are considered as important influences on the composition of Earth’s early atmosphere, with substantial implications for biological evolution. The physicochemical behavior of sulfur-bearing minerals during such natural impact events is crucial for understanding the role of sulfur in the evolution of Earth’s ocean-atmosphere system. Here, we conducted shock recovery experiments on natural pyrite (FeS2) under shock pressures of approximately 20 and 55 GPa to investigate its decomposition characteristics under high-velocity impact. The experimental results indicate that pyrite undergoes partially decomposition into pyrrhotite and sulfur at the shock pressure of about 55 GPa, revealing its thermodynamic instability and the release of sulfur vapor. Our results suggest that the desulfurization of pyrite during meteorite impacts may contribute to the release of sulfur gases into oceanic and atmospheric systems. Such environmental changes may be linked to the Permian-Triassic mass extinction event approximately 250 million years ago, providing important insights into the biological crises of that era.
-
Key words:
- pyrite /
- shock recovery /
- sulfur degassing /
- ocean-atmosphere /
- bio-extinction
-
在地球漫长的演化过程中,陨石/小行星(陨星)撞击事件曾频繁发生。这些事件不仅塑造了地球的表面特征,而且对其地质结构、气候变迁以及生物演化产生了深远的影响[1–4]。位于南非的弗里德堡撞击坑(Vredefort,约2.02×109 年)[5–6]和墨西哥湾的希克苏鲁伯撞击坑(Chicxulub,约6.6×107 年)[7–8]作为现存的2个显著实例,体现了这类事件对地球大气形成和演化的重要性。其中,希克苏鲁伯撞击事件被认为是导致白垩纪-古近纪生物大灭绝的关键因素之一[8]。研究表明,该事件导致地球表面和陨石内部的含碳矿物及含硫矿物向大气排放的CO2和含硫气体通量分别达到(425±160) Gt和(325±130) Gt,使全球气温剧烈变化,并持续超过10万年[9–10]。
硫是地球系统中最重要的挥发分元素之一,广泛分布并循环于各个圈层[11–13]。硫拥有从−2到+6的多种价态形式,在陨石撞击过程中可能释放出硫化氢(H2S)、硫蒸气(S2)、二氧化硫(SO2)和三氧化硫(SO3)等具有强毒性和高活性的含硫气体,这些气体不仅可以直接与大气成分发生反应并改造大气,而且可能形成硫酸盐气溶胶,引发全球气候剧烈变化。但值得注意的是,含硫气体对大气和气候改造的能力各不相同。例如,硫酸盐分解可生成SO2和SO3。SO3可直接生成硫酸气溶胶;而SO2需先被氧化成SO3,再与水反应形成硫酸气溶胶,这一过程相对缓慢且气溶胶颗粒更小,对气候的影响更显著[14–15]。因此,研究含硫矿物在陨石撞击过程中的脱气行为,对于了解地球大气的演化过程和规律、解释地球历史上发生的一些重大气候变化和生物灭绝现象至关重要。
地球历史上比白垩纪-古近纪生物大灭绝更早的二叠纪末期(约2.5×108 年)可能出现过一次更为严重的生物危机,即二叠纪末大灭绝(end-Permian mass extinction,EPME)事件[16–18]。位于澳大利亚的Bedout构造[19]表明,这一时期可能发生过与希克苏鲁伯类似的撞击事件,向大气中释放了大量的含碳气体和含硫气体。然而,含硫矿物在二叠纪撞击事件期间是否存在类似碳酸盐矿物的脱气现象,脱气产物和种类具体是什么,与生物大灭绝事件是否存在关联等问题,尚需进一步的研究来明确。
黄铁矿(FeS2)作为二叠纪末期广泛分布于陆地和海洋中的代表性含硫矿物[20–22],其冲击脱气行为对理解二叠纪-三叠纪边界(Permian-Triassic boundary,PTB)的生物灭绝事件以及其他类地行星(如火星)的大气和气候演化规律至关重要。已有实验研究表明,黄铁矿在大气压力环境下加热至约800 K时会发生歧化分解反应,转换为磁黄铁矿并释放单质硫[23–25]。随着压力增大,黄铁矿的分解温度以约90 K/MPa的速率增加,但研究压力局限于7 GPa[26]。在更高的压力条件下(10~15 GPa),黄铁矿与石英混合物的高压脉冲冲击加载实验结果显示,冲击后样品间隙中形成了以微小液滴和发丝细脉形式存在的熔融磁黄铁矿[27]。在冲击压力约为22 GPa的实验中,尽管明确了冲击载荷会增加黄铁矿缺陷的数量从而可能导致其热稳定性变化,但并未观察到明确的结构转变与脱气现象[28–29]。在相似的压力条件下(25 GPa),Sclar等[30]对单晶黄铁矿进行了冲击回收实验,清晰地观察到受冲击诱导形成的磁黄铁矿。Hugoniot状态方程研究表明,黄铁矿在28.9~38.5 GPa的冲击压力范围内,密度随冲击压力增大而增加,可能存在未知的高压相[31],但在高达120 GPa的冲击压缩实验中并未发现类似现象[32–33]。上述研究表明,虽然已经对黄铁矿的冲击响应开展了大量实验研究,但其在冲击载荷下的物理化学行为仍然存在争议,特别是在冲击加载下是否会出现由冲击波引起的脱硫和分解现象仍需进一步探讨。
高速撞击回收实验技术是一种有效模拟矿物在自然环境中遭受陨石撞击冲击压缩情况的技术,已被广泛应用于研究含水矿物、含碳矿物和含硫矿物等含挥发分矿物(如方解石[34–35]、多孔砂岩[36] 、蛇纹石[37]、石膏[38–39]等)的冲击脱气现象和分解行为。本研究将采用冲击回收实验方法,在高约55 GPa的冲击压力下研究天然黄铁矿的热稳定性和脱挥发行为,即利用二级轻气炮发射高速飞片(km/s量级)撞击样品,模拟自然陨石撞击事件中的极端冲击条件[40],分析回收样品并解析样品的冲击响应特性。实验数据将为理解陨石撞击事件对地球表面含硫矿物的影响及其对大气和气候的潜在影响提供关键证据,尤其在二叠纪晚期的全球气候变化以及约2.5亿年前生物灭绝事件的背景下具有重要科学意义。
1. 材料和方法
1.1 实验样品
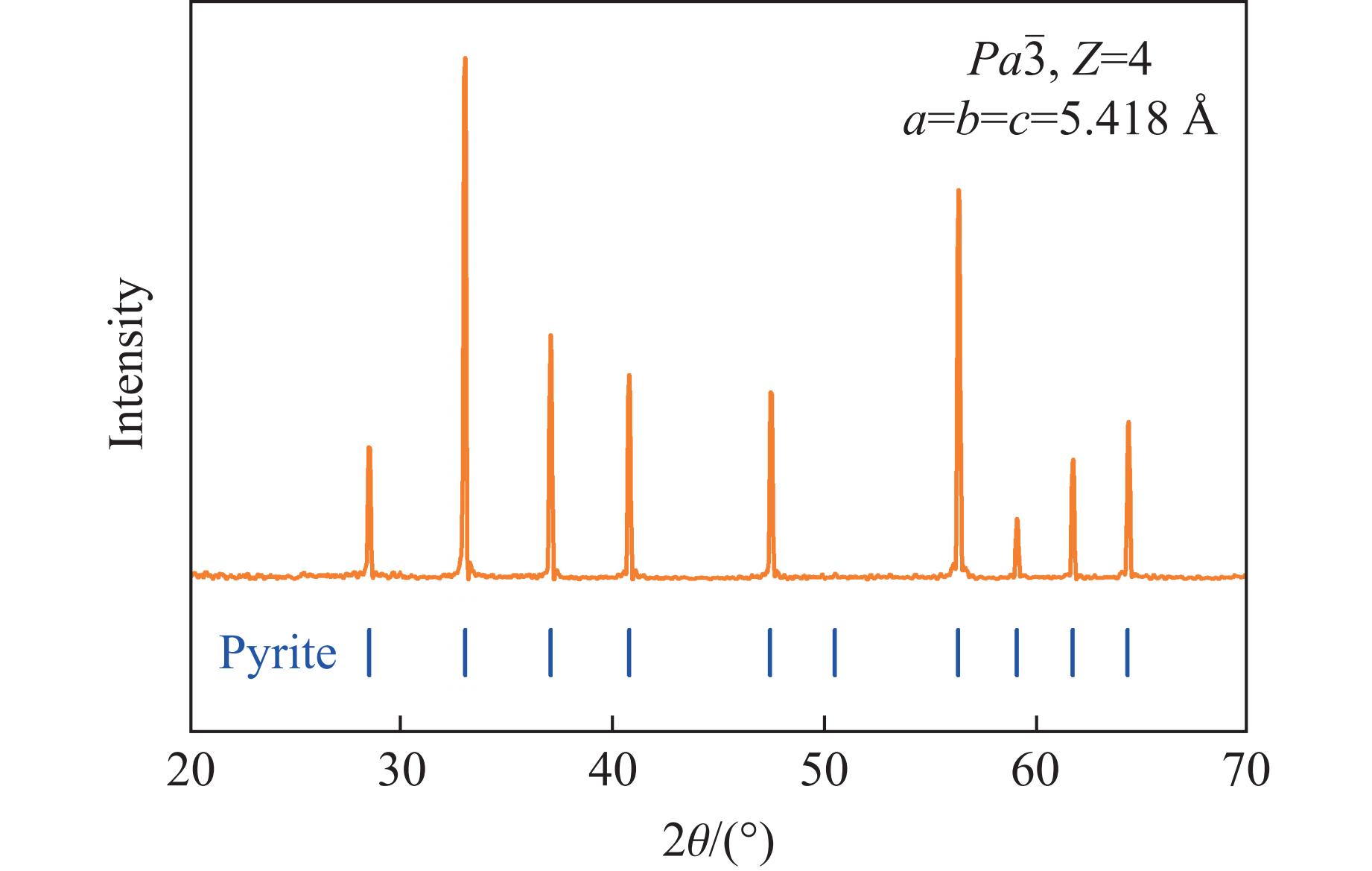
选取天然单晶黄铁矿作为制作冲击回收实验样品的原材料。天然黄铁矿被切割制作成直径约12 mm、厚度约1.5 mm的薄片,随后对其表面进行精细抛光处理。为了明确样品中孔隙率与成分不均匀性对冲击实验结果的影响,通过X射线衍射(X-ray diffraction,XRD)、扫描电子显微镜(scanning electron microscope,SEM)、能量色散谱仪(energy dispersion spectrometer,EDS)和电子探针显微分析仪(electron probe microana,EPMA)对样品进行了详细的成分分析和形貌表征。如图1所示,XRD图谱表明,样品中仅存在立方晶体结构的黄铁矿相,晶胞体积为159.0 Å3,属于等轴晶系,空间群Pa3,未检测到任何其他杂相衍射峰,其中,a、b、c为晶格常数。背散射电子(backscattered electron,BSE)和EDS分析结果显示,样品成分均匀,Fe和S元素在样品表面均匀分布(图2);EPMA定量分析表明,样品化学组成主要为FeS2(质量分数为98.50%),仅检测到少量SiO2杂质(质量分数低于1.50%),见表1。SEM分析结果显示,样品表面无明显缺陷或孔隙。采用阿基米德排体积法测得实验样品的密度为(4.981±0.050) g/cm3,在考虑杂质的影响下,与XRD数据得到的理论密度5.001 g/cm3相当,表明样品内部与表面相同,不存在明显缺陷或孔洞。上述表征结果表明,实验样品的纯度较高,在结构和成分上具有高度的均匀性,满足冲击回收实验对实验样品的需求。
表 1 天然黄铁矿样品的化学成分Table 1. Chemical composition of natural pyrite sampleNo. Mass fraction/% FeS2 Others 1 98.63 1.37 2 98.52 1.48 3 98.35 1.65 Average 98.50 1.50 1.2 冲击回收实验
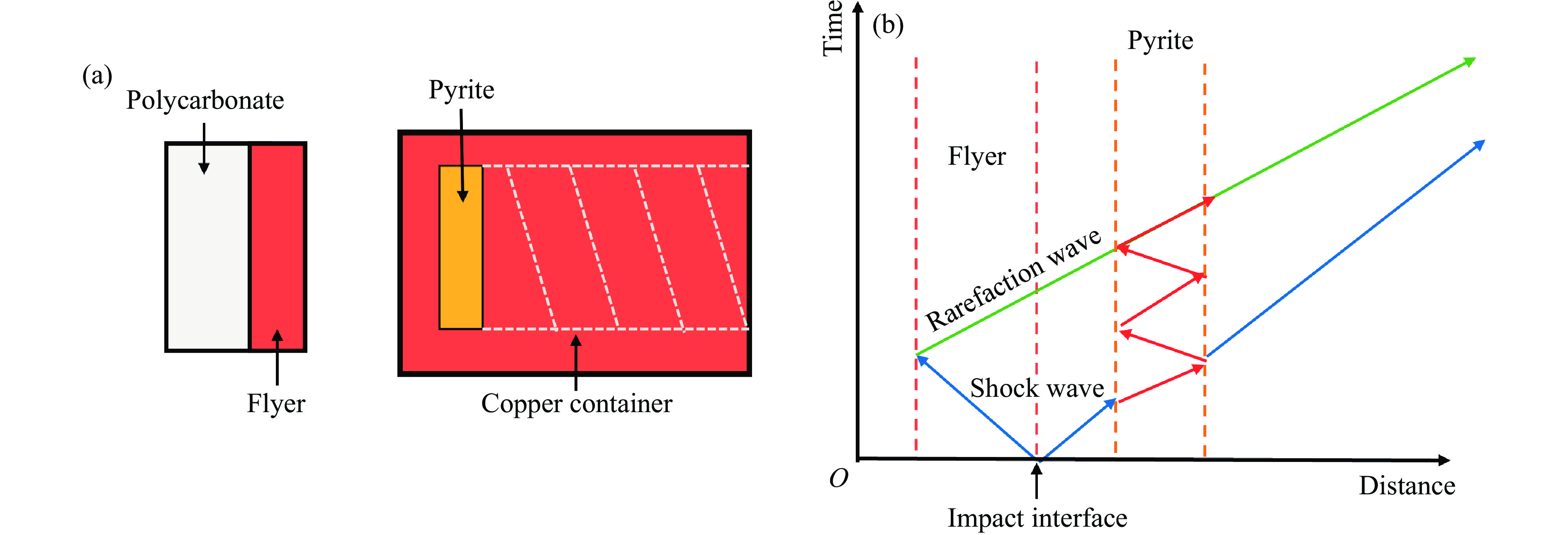
冲击回收实验基于四川大学原子与分子物理研究所一、二级轻气炮实验平台开展,实验装置如图3(a)所示。黄铁矿样品被封装于铜制回收筒内,与碰撞面的距离约3 mm。实验飞片材质采用过渡金属铜(Cu)和钽(Ta),飞片的直径和厚度分别为24和4 mm,固定于聚碳酸酯弹托端部,形成实验弹丸。实验过程中,使用一、二级轻气炮驱动弹丸,使飞片与回收筒发生高速平面碰撞。飞片的撞击速度通过电磁测量法获得,不确定度约为0.5%。
高速碰撞时,飞片与回收筒将产生两列相反方向传播的一维平面冲击波。冲击波在样品和回收筒样品腔中的传播过程如图3(b)所示。一列冲击波在飞片中向后传播,而另一列则在回收筒内向前传播。由于样品的冲击阻抗低于回收筒的冲击阻抗(表2[32, 41–45]),前向传播的冲击波进入样品时仍能保持其冲击波特性,但冲击压力会从容器内的p降至进入样品后的p1。当冲击波穿过样品到达样品与回收筒样品腔的交界面时,将形成反射冲击波,对样品进行二次冲击压缩。因此,样品经历了初始冲击波加载以及多次样品腔内壁反射冲击加载,导致样品内部的冲击压力不断升高。同时,向后传播的冲击波到达飞片后界面时,将反射一列向样品方向传播的稀疏波,稀疏波传播至样品后将导致样品中的压力降低。本研究中的飞片厚度明显大于样品厚度,确保样品可以被冲击波多次加载至与回收筒内部压力平衡的状态。因此,利用测量的表2中的飞片速度以及回收筒、飞片和样品的Hugoniot参数(不确定度小于 1.5%),通过阻抗匹配法可计算得到样品内部的峰值压力[45–46]。表2中,ρ0为零压密度,C0和λ为Hugoniot拟合参数。冲击波首次加载样品后的冲击压力p1以及样品内部最终的峰值压力p见表3,其中:w为飞片撞击速度。共进行了2发冲击回收实验,Ta飞片的撞击速度为0.79 km/s(S1实验,一级轻气炮驱动),Cu飞片的撞击速度为2.20 km/s(S2实验,二级轻气炮驱动),对应的样品内部峰值冲击压力分别为19.7和54.7 GPa。
表 3 冲击回收实验条件Table 3. Conditions for shock recovery experimentsShot No. Flyer Sample container w/(km·s−1) ρ0/(g·cm−3) p1/GPa p/GPa S1 Ta Cu 0.793(4) 4.989 16.3 19.7(3) S2 Cu Cu 2.197(11) 5.010 45.3 54.7(9) 冲击实验完成后,将装有样品的回收筒进行回收。利用机床将回收筒切开后,成功回收到经过冲击高温高压作用后的样品。将部分回收样品通过玛瑙研钵充分研磨成粉末后,使用X射线衍射仪(日本理学 Ultima Ⅳ型,Cu靶,电压40 kV,电流30 mA)对冲击后样品的相组成进行测试分析。此外,使用同步热分析仪(德国耐驰STA449F5)对初始样品和回收样品进行热特性分析,进一步探究冲击加载对黄铁矿热稳定性和分解量的影响。最后,采用SEM对回收样品的微观形貌进行了详细观察,分析黄铁矿在冲击过程中是否发生了显著的变质效应以及明确黄铁矿的冲击脱气行为。
2. 实验结果
2.1 物相分析
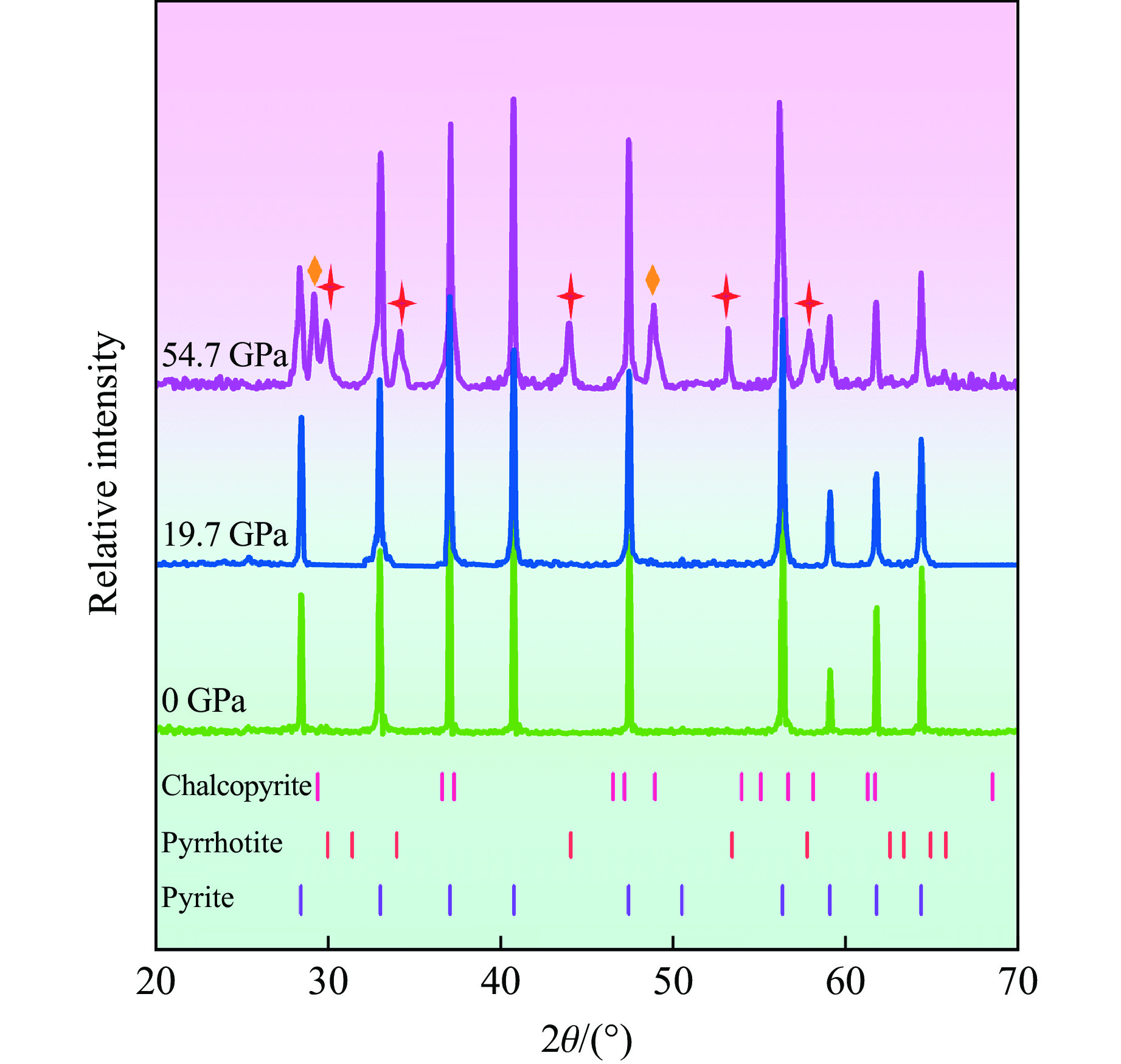
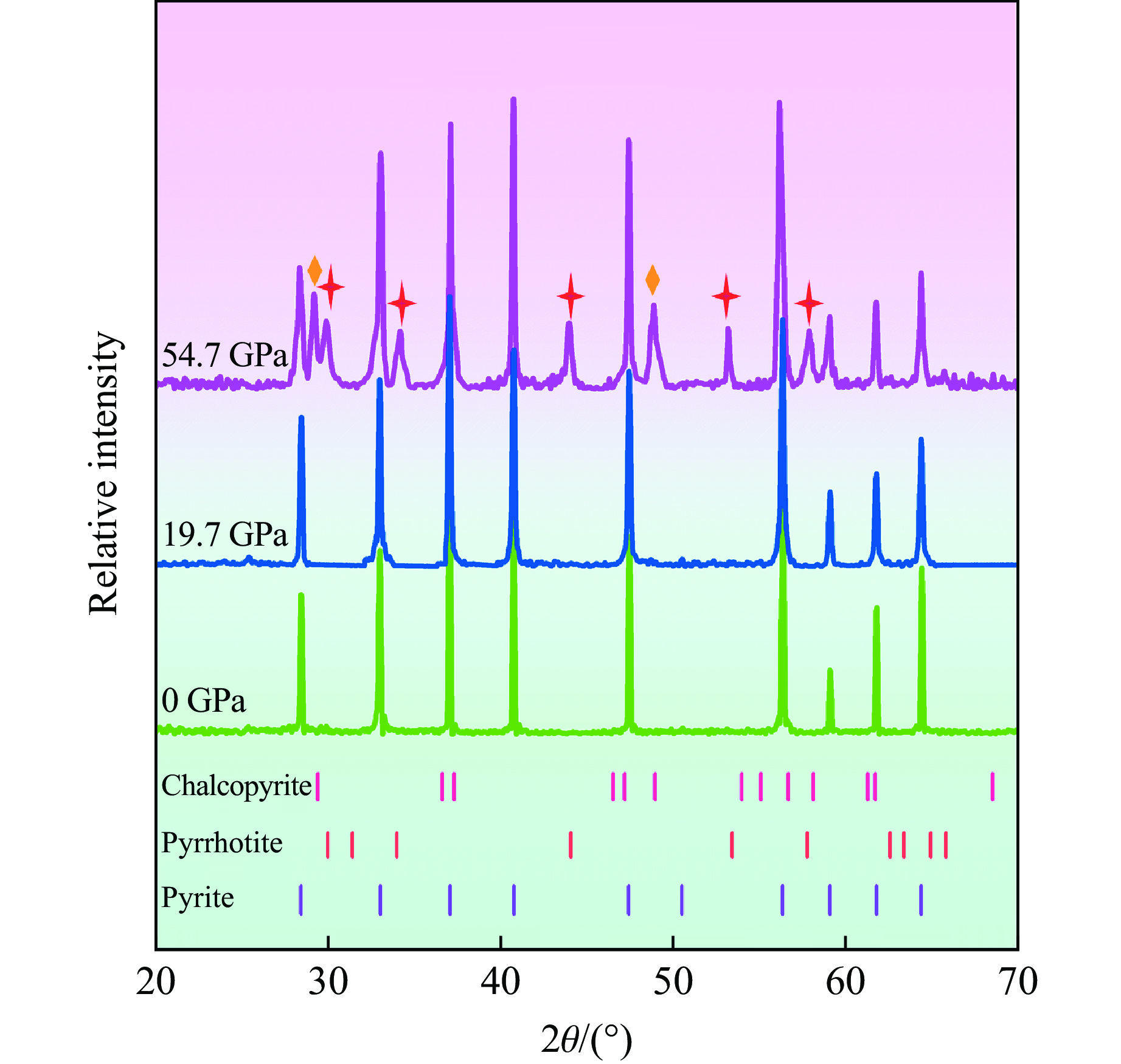
图4展示了初始黄铁矿样品和冲击回收样品的XRD谱,图中红色十字星形标注了除黄铁矿外新相磁黄铁矿的衍射峰,橙色菱形标记了黄铜矿的衍射峰位置,浅橙色、浅红色和浅紫色的小竖线分别表示国际衍射数据中心(International Centre for Diffraction Data,ICDD)PDF2数据库中的磁黄铁矿、黄铜矿和黄铁矿标准衍射峰所在位置。实验结果表明,当样品承受19.7 GPa冲击压力后,回收样品的衍射峰与初始黄铁矿样品高度一致,未出现任何新的衍射峰,表明在此冲击压力下黄铁矿保持稳定,未发生分解反应。然而,当飞片撞击速度增加至2.20 km/s时,对应样品承受的冲击压力达到54.7 GPa,回收样品的XRD谱与19.7 GPa回收样品相比发生了明显变化。虽然部分黄铁矿衍射峰的强度略有下降,但其主要晶相依然存在,表明黄铁矿在54.7 GPa冲击压力下仍作为主相存在。将30°~60°衍射角范围内新增的7个衍射峰与标准衍射峰对比发现,其中5个衍射峰来自磁黄铁矿,与常压下高温实验中黄铁矿的热分解产物[23–24]一致。此外,54.7 GPa冲击压力下获得的回收筒表面出现了部分破裂和孔洞,表明冲击过程可能伴随气体释放。由此可知,黄铁矿在54.7 GPa冲击压力下部分分解为磁黄铁矿,并释放了含硫气体。值得注意的是, 29.48°和48.90°衍射角处还出现了黄铜矿的2个衍射峰。由于黄铜矿的衍射峰数量较少,且不属于Fe-S二元体系,推测黄铜矿可能是位于撞击边缘部分的黄铁矿与铜回收筒反应或者黄铁矿分解产物与铜回收筒反应后形成的。
黄铁矿中Fe和S元素的化合价分别为+2价和−1价。虽然磁黄铁矿中Fe的化合价会因为部分Fe原子的缺失而发生微小变化,但整体价态保持+2价, S则为−2价[47]。考虑到分解过程中的价态和元素平衡,黄铁矿的冲击分解过程可以通过以下反应式描述
(1−x)FeS2==Fe1−xS+(1−2x)S (1) 2.2 微观形貌分析
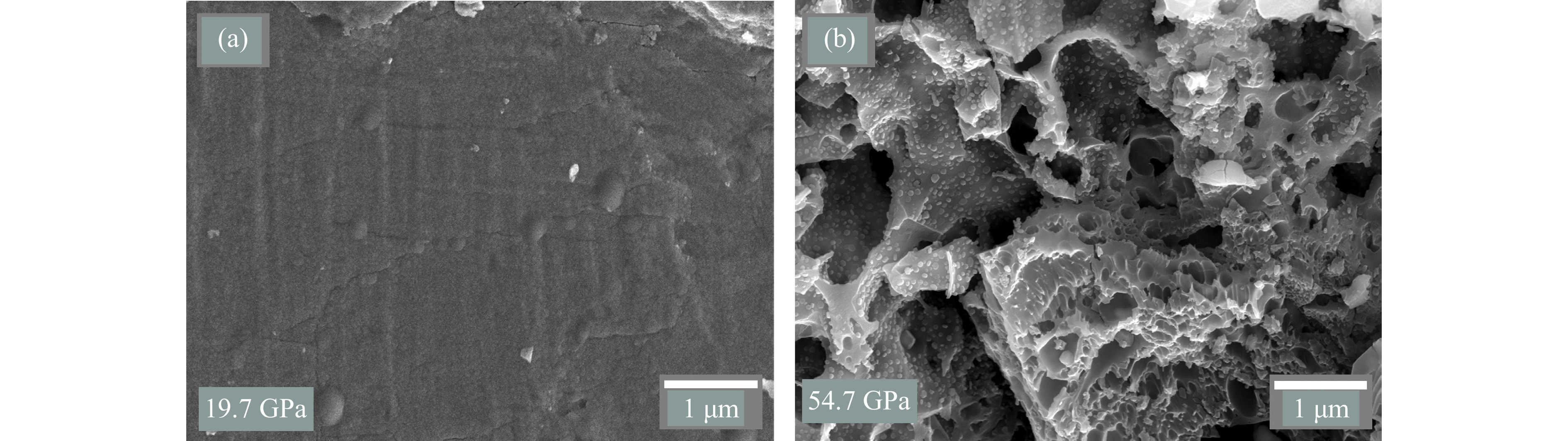
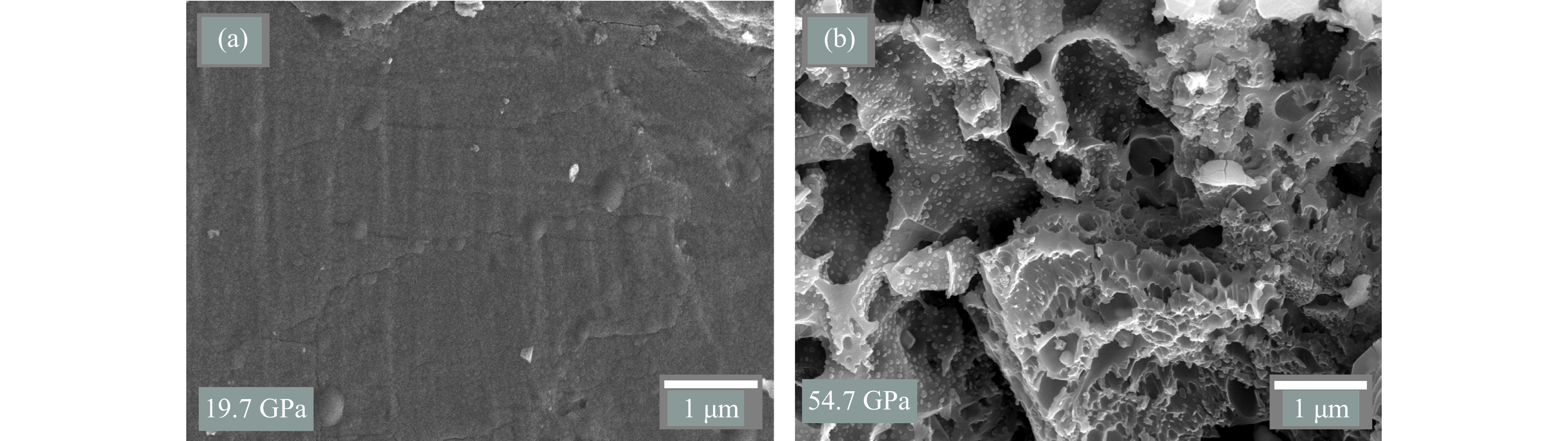
SEM被广泛应用于冲击回收矿物的微观形貌分析,在一些典型的冲击实验中,矿物表面的形态变化可以提供冲击导致相变或分解行为的直观证据[48]。因此,利用SEM对黄铁矿冲击后的回收样品表面进行了微观形貌分析。图5(a)显示了19.7 GPa冲击压力下回收样品的SEM图像,与未受冲击的样品(图2(a))相比,回收样品颗粒表面较为平整,无明显孔洞,仅局部区域出现了微小裂纹,表明在19.7 GPa冲击压力下,黄铁矿样品主要经历了机械和物理变形,未表现出分解、放气或熔化等现象,与XRD分析结果一致。类似的观察结果在磁黄铁矿的冲击实验中也得到了证实。Mang等[49]在8 GPa冲击压力下观察到磁黄铁矿仅出现了局部裂纹,尽管在20 GPa冲击压力下微裂纹的数量明显增多,但并未发现其他显著的微观结构变化。此外,锆石冲击实验[50]表明,在20 GPa冲击压力条件下,锆石中普遍存在位错和大量微裂纹,但同样未观察到其他明显的微观结构特征。这些结果表明,较低的冲击压力可能达不到某些矿物的分解阈值。
图5(b)为冲击压力增大至54.7 GPa时的微观结构变形 SEM图像。样品表面出现了孔洞,并且孔洞内部还密集分布着小气泡,类似于其他含硫矿物冲击回收实验中观察到的形貌。例如,Rao等[51]在31 GPa冲击压力下的玄武岩与硫酸铁混合物回收样品中观察到了大量圆形和细长形的富硫气泡。此外,本研究还在冲击实验回收样品中观察到这些气泡与shergottite撞击熔体中发现的富硫气泡簇[52]类似。研究发现,在多次冲击压缩作用下,黄铁矿的S―Fe键可能断裂并在FeS2基质中形成了更强的S―S键,产生单质硫蒸气[53]。这些气体的不断产生和积聚导致冲击样品表面和内部发生膨胀,进而形成气泡结构,气泡内的压力不断上升,在达到气泡破碎的临界压力前,这些气体不会被释放出来。此外,回收样品的所有区域均出现了形状和大小不一的孔洞结构,可能是气泡大规模破裂导致的结果。Lange等[54]基于蛇纹石冲击回收实验结果推测,矿物在冲击压力释放过程中分解出的挥发性气体的散失是导致不规则孔洞形成的主要原因。根据54.7 GPa回收样品的形貌可推测,黄铁矿不仅可能在冲击压缩过程中发生分解和脱气,也可能在冲击卸载阶段,受到残留温度的作用,导致其亚稳态样品进一步分解、脱气[55–56]。
冲击压缩过程中,系统与外界环境之间几乎不进行热交换,可以近似视为绝热压缩过程。因此,冲击产生的能量转化为内能后无法迅速释放,从而在样品内部累积[57–59]。在冲击卸载阶段,温度的下降速率远低于压力的下降速率[56, 60–61],这种情况不仅导致结构缺陷的产生,还可能导致样品处于不稳定的亚稳态[62–64]。单次冲击实验表明,黄铁矿在120 GPa冲击压力范围内依然处于热力学稳定状态[32–33]。然而,本研究中的冲击实验发现,黄铁矿在55 GPa已经分解、脱气。因此,黄铁矿在冲击回收实验条件下的分解可能发生在冲击卸载阶段。黄铁矿分解产生的含硫气体在样品内部积聚后无应力约束而自由释放,形成了无规则孔洞和微小气泡的微观形貌特征。
2.3 热特征分析
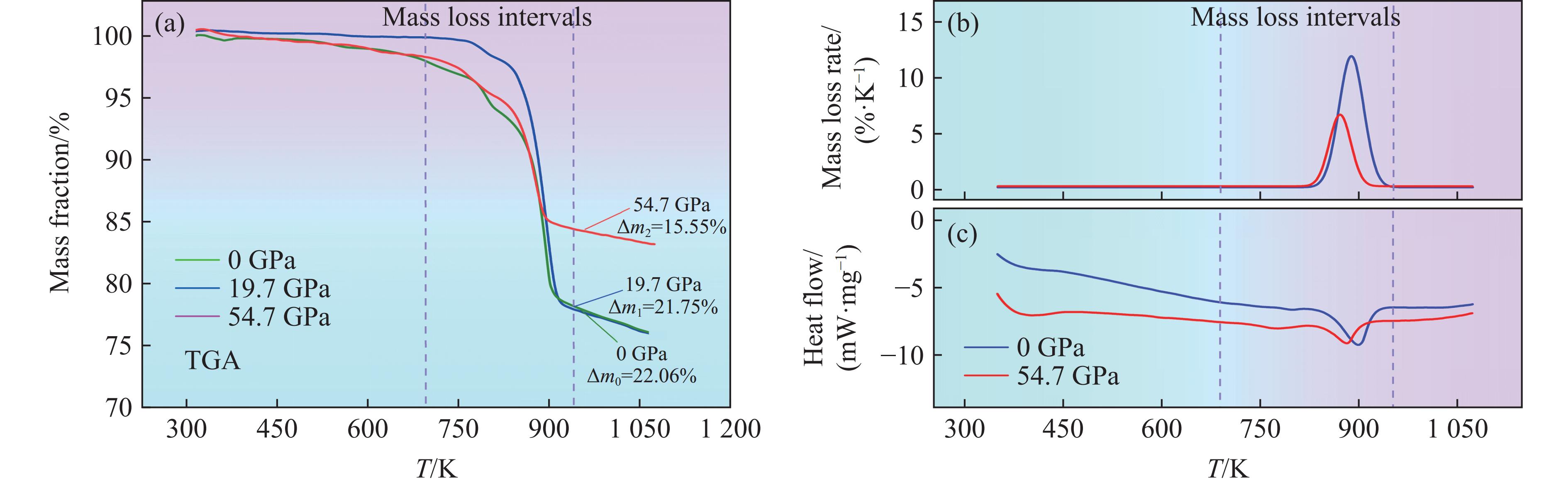
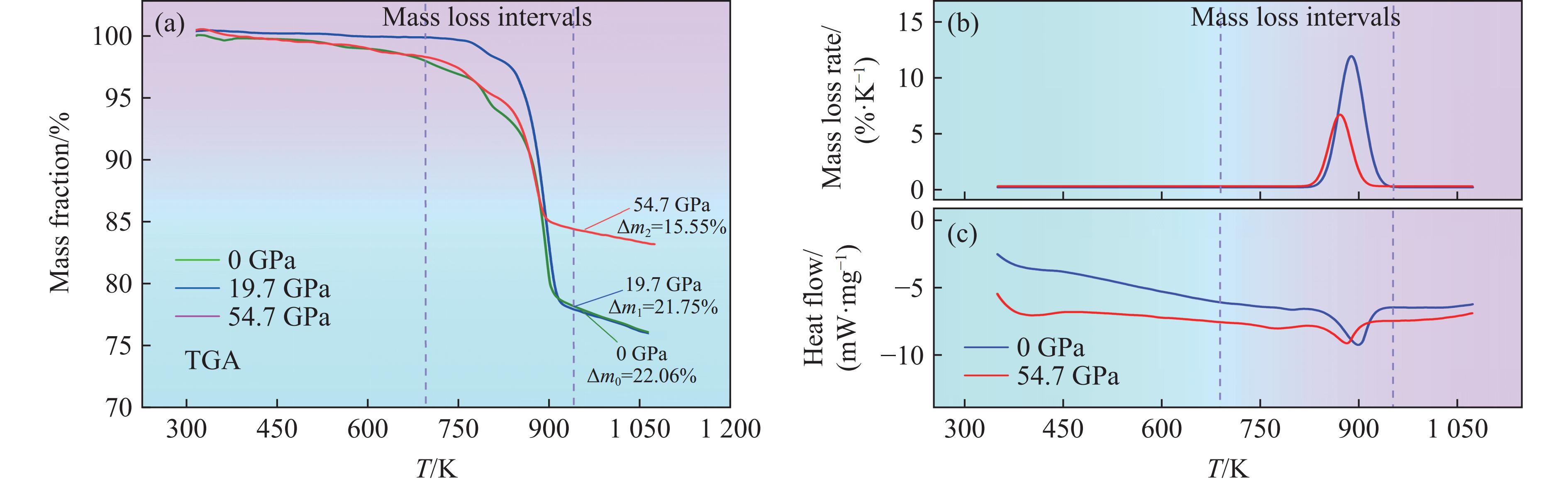
热特征分析是确定回收样品中残余初始样品质量的关键。本研究对初始黄铁矿以及冲击后的回收样品进行了热特性测试,包括热重分析(thermogravimetric analysis,TGA)、微商热重(derivative thermogravimetric,DTG)分析和差示扫描量热(differential scanning calorimetry,DSC)分析,测试温度范围为298~
1073 K,升温速率为10 K/min,在氩气气氛下进行,结果如图6所示。TGA结果显示,初始黄铁矿样品在750~900 K时的质量损失率(Δm0)为22.06%,这是黄铁矿在高温下分解为磁黄铁矿并释放硫蒸气导致的,该结果与Xu等[25]开展的热特性分析结果一致,也与根据化学式计算的理论失重结果吻合。由于回收样品在冲击实验中已预先经历了分解和脱硫过程,因此在回收样品中观察到的质量损失全部是由未分解的黄铁矿产生的。对于19.7 GPa压力下的回收样品,失重率(Δm1)为21.75%,与初始样品在实验误差允许的范围内相当,表明冲击并没有引起黄铁矿分解,与XRD和SEM测试分析结果一致。然而,当冲击压力增大至54.7 GPa时,回收样品的失重率(Δm2)显著降低15.55%,表明黄铁矿在冲击高温高压下已发生部分分解,TGA和DSC分析进一步验证了该结论。冲击后样品的DTG曲线显示热分解过程中吸热峰值降低,表明硫的释放显著减少。同样,DSC分析发现冲击后样品的吸热量明显小于初始样品,进一步证明黄铁矿在54.7 GPa压力下发生了部分分解。通过将回收样品的失重率与初始样品比较,得到黄铁矿在54.7 GPa冲击压力条件下的分解率为29.5%,说本实验的压力范围还未达到黄铁矿完全分解的阈值,需要通过进一步的实验来确定。然而,54.7 GPa压力下回收筒碰撞面已经出现裂纹和排气洞,因此,在更高压力下进行冲击回收实验需要克服回收容器材料耐受性的限制。后续将设计更加完善的实验来探究这个问题。3. 讨论及应用
PTB沉积物中发现的富勒烯(C60~C200)内封闭的氦和氩同位素情况显示,地球在约2.5亿年前可能经历了陨石或彗星(小行星)撞击事件[19, 65],这类超高速撞击通常会引发许多矿物(如方解石、针铁矿等)发生脱碳和脱水等变质效应[48, 66]。同样,在这种撞击事件中,含硫矿物(如黄铁矿)可能也会经历类似的物理与化学转变。
按照撞击强度可将陨石撞击产生的压力分为6个阶段(阶段1~阶段6),该分类方案主要用于量化陨石撞击对地质物质的影响程度[67–68]。随着冲击压力的增加,岩石和矿物的变质效应逐渐加剧。阶段1的冲击压力较低(小于5 GPa),岩石和矿物仅出现细微裂缝,没有明显的变质迹象。随着进入更高阶段,冲击变质特征愈加显著。例如,火星陨石中的多孔陨硫铁(FeS)在阶段6的冲击压力(55~90 GPa)下出现明显的脱硫,形成了气泡和奶酪状微观特征[69–70]。这种现象与本研究中从55 GPa冲击压力下回收样品展现出的气泡和网格状孔洞结构极为相似(图5),表明黄铁矿和陨硫铁在陨石撞击过程中都可能发生类似的脱硫反应。
陨石的冲击阻抗是评估自然撞击事件中瞬时压力状态的关键参数。在众多陨石类型中,铁陨石和普通球粒陨石较为常见。Dai等[71]通过冲击实验研究南丹铁陨石(密度为7.780 g/cm³)和吉林普通球粒陨石(密度为3.469 g/cm³)的状态方程,研究结果显示,这2类陨石的冲击阻抗均低于本研究中选用的Ta和Cu飞片的冲击阻抗。因此,当陨石以较低撞击速度(低于使黄铁矿发生分解反应的飞片速度)撞击地表时,黄铁矿有可能保持其热力学稳定性。以吉林球粒陨石为例,如果其撞击速度显著低于3.8 km/s,即对应的冲击压力远低于54.8 GPa时,这种情况下黄铁矿可能不会发生分解反应。值得注意的是,陨石的撞击速度通常超过数千米每秒[72–73],最小撞击速度由被撞星体的逃逸速度量度。例如,火星上陨石的最小撞击速度约为5 km/s,而地球上的撞击速度高达11.2 km/s[74–75]。尽管存在罕见的低速球粒陨石(小于4.5 km/s),但其速度仍远高于本实验中黄铁矿发生脱气反应时对应的撞击速度(约为2.2 km/s)。此外,数值模拟结果显示,直径约10 km的小行星以20 km/s的速度撞击地球,撞击深度可达30 km,30 s内可形成半径约为60 km的撞击坑,并喷出约50 Tt海洋物质和35 Tt地壳物质[76–77]。虽然板块构造和侵蚀等地质过程导致陨石撞击痕迹逐渐消失,但随着地质学分析技术的发展,地球历史上的陨石撞击事件有望被揭露。假如二叠纪末期确实发生了陨石撞击事件,那么位于大陆和海洋地壳中的黄铁矿可能经历了冲击分解过程,释放出大量的硫。
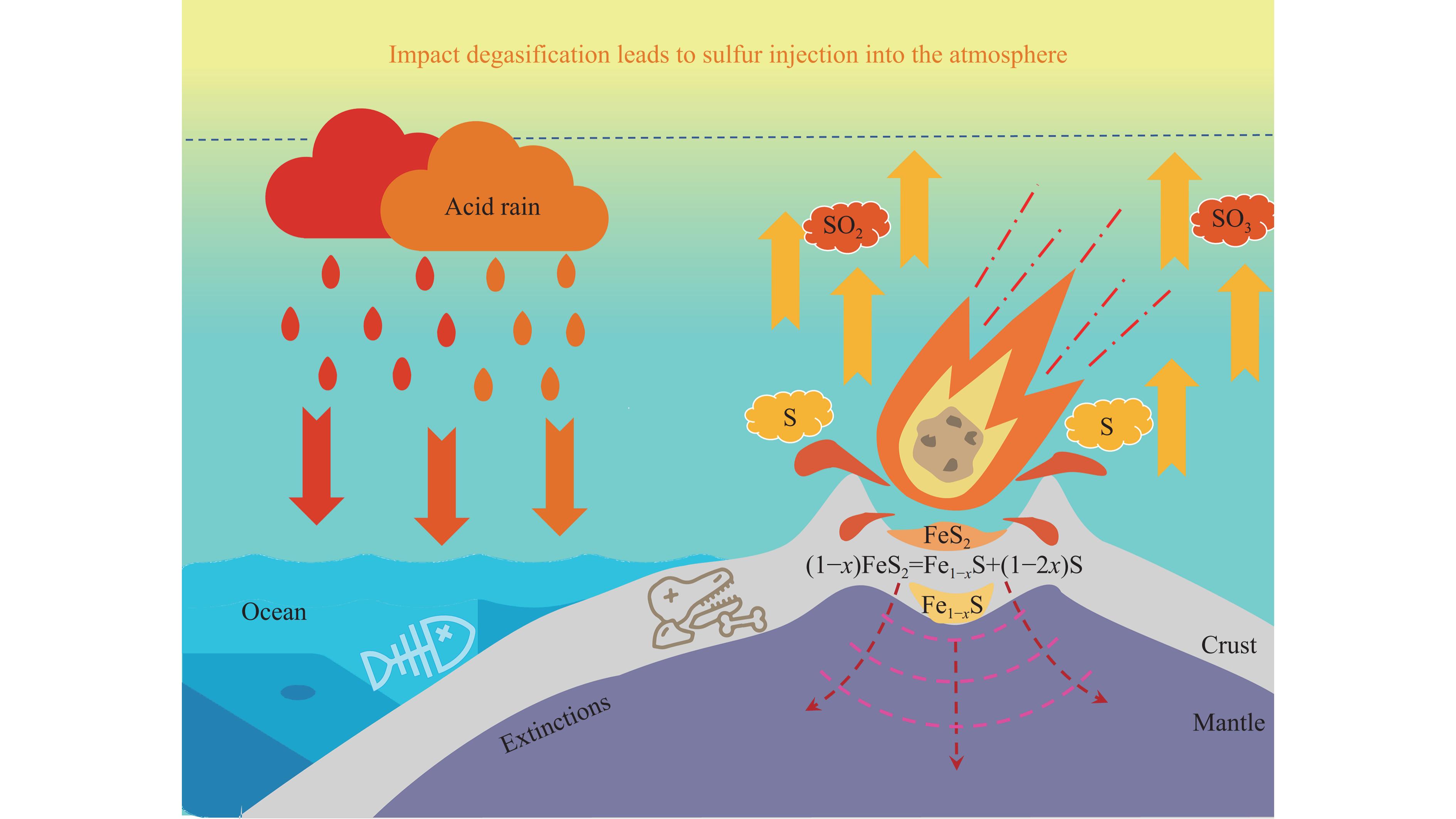
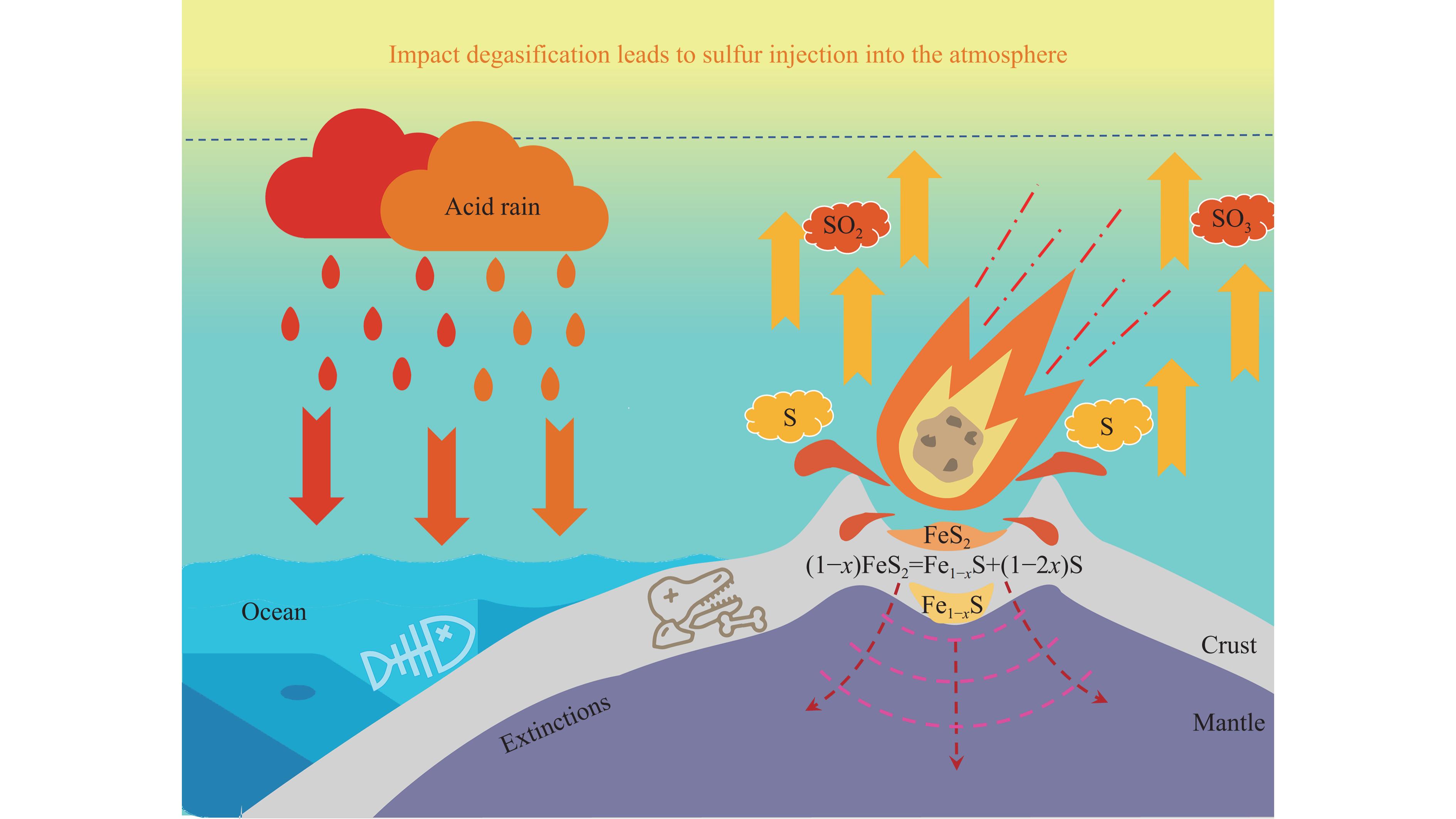
Li等[78]对澳大利亚悉尼盆地的硫同位素进行了研究,结果显示,地壳和地幔中的硫可能被大量释放到海洋-大气系统中[16, 79–80]。由于单质硫的沸点较低,且撞击瞬间造成的压力可能高达数十吉帕到数百吉帕,并伴随极高的冲击温度[77, 81–82],因此,黄铁矿分解形成的单质硫可能会迅速蒸发并被氧化成SO2和SO3等酸性气体,这些气体再与大气中的H2O或OH-基团反应可生成硫酸(H2SO4)。一方面,H2SO4与H2O反应可以凝结成核,最终形成具有辐射活性的硫酸气溶胶,这些气溶胶在对流层中的寿命通常较短,仅为数周。然而,当这些气溶胶进入平流层后,其寿命可延长至一年甚至数年[83]。硫酸气溶胶粒子能够有效散射太阳辐射[84],减少到达地面的太阳能量,导致地表温度下降,进而可能引发全球气温的急剧下降[85–86]。此外,硫酸气溶胶还可以作为催化剂,激活大气中的氯,增强其对臭氧分子的破坏作用[87–88],导致大气臭氧浓度下降,尤其是在极地地区[89]。因此,硫酸气溶胶的存在可能对大气臭氧层的消耗和变薄产生重要影响[90–91]。Kaiho等[16]对来自中国南部煤山剖面的样品进行了硫同位素分析,结果表明,二叠纪末期海洋中碳酸盐矿物结构晶格中的硫酸盐的硫同位素值(δ34S )显著下降,这种异常结果为地外撞击事件引发的海洋-大气“硫注入”假说提供了支持[16, 79]。另一方面,H2SO4可溶解于H2O,并随降雨返回地面,形成硫酸型酸雨[92–93],如图7所示,这一过程可能导致海洋酸化。最近对腕足类贝壳进行的硼同位素值(δ11B)分析表明,海水pH 值的大幅下降与二叠纪末期大灭绝事件开始时间吻合,进一步证明了海水酸化与该时期大灭绝事件之间的紧密联系[94]。综上所述,黄铁矿在撞击事件中的脱气反应为大规模硫释放提供了潜在的地质机制,并为二叠纪末期的海洋-大气硫循环提供了可能的路径,这一发现不仅有助于深入理解PTB生物灭绝,也为未来地质和行星撞击研究提供了重要的参考依据。
4. 结 论
通过冲击回收实验,研究了天然黄铁矿在受到类似陨石撞击条件下的分解与脱气行为。实验结果显示,在约55 GPa的冲击压力下,黄铁矿发生了部分分解,生成了磁黄铁矿和单质硫。这一发现表明,在小行星或陨石撞击事件中,地表和海底的黄铁矿可能经历类似的分解过程,释放出大量含硫气体,进而对早期地球表层大气和海洋环境产生显著影响,这种由撞击引发的剧烈环境变化可能为二叠纪末期生物大灭绝事件提供了新的线索和理论支持。
-
表 1 天然黄铁矿样品的化学成分
Table 1. Chemical composition of natural pyrite sample
No. Mass fraction/% FeS2 Others 1 98.63 1.37 2 98.52 1.48 3 98.35 1.65 Average 98.50 1.50 表 3 冲击回收实验条件
Table 3. Conditions for shock recovery experiments
Shot No. Flyer Sample container w/(km·s−1) ρ0/(g·cm−3) p1/GPa p/GPa S1 Ta Cu 0.793(4) 4.989 16.3 19.7(3) S2 Cu Cu 2.197(11) 5.010 45.3 54.7(9) -
[1] GRIEVE R A F. The record of impact on Earth: implications for a major Cretaceous/Tertiary impact event [M]//SILVER L T, SCHULTZ P H. Geological Implications of Impacts of Large Asteroids and Comets on the Earth. Boulder: Geological Society of America, 1982: 25−38. [2] RAUP D M. Biological extinction in earth history [J]. Science, 1986, 231(4745): 1528–1533. doi: 10.1126/science.11542058 [3] JUNIUM C K, ZERKLE A L, WITTS J D, et al. Massive perturbations to atmospheric sulfur in the aftermath of the Chicxulub impact [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(14): e2119194119. doi: 10.1073/pnas.2119194119 [4] SCHMIEDER M, KRING D A. Earth’s impact events through geologic time: a list of recommended ages for terrestrial impact structures and deposits [J]. Astrobiology, 2020, 20(1): 91–141. doi: 10.1089/ast.2019.2085 [5] GAY N C. Spherules on shatter cone surfaces from the vredefort structure, South Africa [J]. Science, 1976, 194(4266): 724–725. doi: 10.1126/science.194.4266.724 [6] ALLEN N H, NAKAJIMA M, WÜNNEMANN K, et al. A revision of the formation conditions of the vredefort crater [J]. Journal of Geophysical Research: Planets, 2022, 127(8): e2022JE007186. doi: 10.1029/2022JE007186 [7] ALVAREZ W, CLAEYS P, KIEFFER S W. Emplacement of cretaceous-tertiary boundary shocked quartz from Chicxulub crater [J]. Science, 1995, 269(5226): 930–935. doi: 10.1126/science.269.5226.930 [8] SCHULTE P, ALEGRET L, ARENILLAS I, et al. The Chicxulub asteroid impact and mass extinction at the cretaceous-paleogene boundary [J]. Science, 2010, 327(5970): 1214–1218. doi: 10.1126/science.1177265 [9] POPE K O, BAINES K H, OCAMPO A C, et al. Energy, volatile production, and climatic effects of the Chicxulub Cretaceous/Tertiary impact [J]. Journal of Geophysical Research: Planets, 1997, 102(E9): 21645–21664. doi: 10.1029/97JE01743 [10] KAWARAGI K, SEKINE Y, KADONO T, et al. Direct measurements of chemical composition of shock-induced gases from calcite: an intense global warming after the Chicxulub impact due to the indirect greenhouse effect of carbon monoxide [J]. Earth and Planetary Science Letters, 2009, 282(1/2/3/4): 56–64. doi: 10.1016/j.jpgl.2009.02.037 [11] VON GEHLEN K. Sulfur in the Earth’s mantle—a review [M]//SCHIDLOWSKI M, GOLUBIC S, KIMBERLEY M M, et al. Early Organic Evolution. Berlin: Springer, 1992: 359−366. [12] WALTERS J B, CRUZ-URIBE A M, MARSCHALL H R. Sulfur loss from subducted altered oceanic crust and implications for mantle oxidation [J]. Geochemical Perspectives Letters, 2020, 13: 36–41. doi: 10.7185/geochemlet.2011 [13] CHOWDHURY P, DASGUPTA R. Sulfur extraction via carbonated melts from sulfide-bearing mantle lithologies-implications for deep sulfur cycle and mantle redox [J]. Geochimica et Cosmochimica Acta, 2020, 269: 376–397. doi: 10.1016/j.gca.2019.11.002 [14] PIERAZZO E, HAHMANN A N, SLOAN L C. Chicxulub and climate: radiative perturbations of impact-produced S-bearing gases [J]. Astrobiology, 2003, 3(1): 99–118. doi: 10.1089/153110703321632453 [15] MORGAN J V, BRALOWER T J, BRUGGER J, et al. The Chicxulub impact and its environmental consequences [J]. Nature Reviews Earth & Environment, 2022, 3(5): 338–354. doi: 10.1038/s43017-022-00283-y [16] KAIHO K, KAJIWARA Y, NAKANO T, et al. End-Permian catastrophe by a bolide impact: evidence of a gigantic release of sulfur from the mantle [J]. Geology, 2001, 29(9): 815–818. doi: 10.1130/0091-7613(2001)029<0815:EPCBAB>2.0.CO;2 [17] BRENNECKA G A, HERRMANN A D, ALGEO T J, et al. Rapid expansion of oceanic anoxia immediately before the end-Permian mass extinction [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(43): 17631–17634. doi: 10.1073/pnas.1106039108 [18] ZHANG H, ZHANG F F, CHEN J B, et al. Felsic volcanism as a factor driving the end-Permian mass extinction [J]. Science Advances, 2021, 7(47): eabh1390. doi: 10.1126/sciadv.abh1390 [19] BECKER L, POREDA R J, BASU A R, et al. Bedout: a possible end-Permian impact crater offshore of Northwestern Australia [J]. Science, 2004, 304(5676): 1469–1476. doi: 10.1126/science.1093925 [20] JIANG Y F, TANG Y G, CHOU C L. Research on genesis of pyrite near the Permian-Triassic boundary in Meishan, Zhejiang, China [J]. Journal of China University of Mining and Technology, 2006, 16(4): 457–460. doi: 10.1016/S1006-1266(07)60047-9 [21] SALISBURY J, GRÖCKE D R, CHEUNG H D R A, et al. An 80-million-year sulphur isotope record of pyrite burial over the Permian-Triassic [J]. Scientific Reports, 2022, 12(1): 17370. doi: 10.1038/s41598-022-21542-4 [22] SHEN W J, LIN Y T, XU L, et al. Pyrite framboids in the Permian-Triassic boundary section at Meishan, China: evidence for dysoxic deposition [J]. Palaeogeography, Palaeoclimatology, Palaeoecology, 2007, 253(3/4): 323–331. doi: 10.1016/j.palaeo.2007.06.005 [23] AHMED M A, ELLID M S, AMAMI S, et al. Mössbauer study of the thermal decomposition of pyrite in one Egyptian coal heated in hydrogen atmosphere at different temperatures (438 K–715 K) [J]. Arabian Journal for Science and Engineering, 2006, 31(2A): 159–165. [24] HU G L, DAM-JOHANSEN K, WEDEL S, et al. Decomposition and oxidation of pyrite [J]. Progress in Energy and Combustion Science, 2006, 32(3): 295–314. doi: 10.1016/j.pecs.2005.11.004 [25] XU H W, GUO X F, SEAMAN L A, et al. Thermal desulfurization of pyrite: an in situ high-T neutron diffraction and DTA-TGA study [J]. Journal of Materials Research, 2019, 34(19): 3243–3253. doi: 10.1557/jmr.2019.185 [26] SHARP W E. Melting curves of sphalerite, galena, and pyrrhotite and the decomposition curve of pyrite between 30 and 65 kilobars [J]. Journal of Geophysical Research, 1969, 74(6): 1645–1652. doi: 10.1029/JB074i006p01645 [27] ZHMODIK S M, VERKHOVTSEVA N V, CHIKOV B M, et al. Experimental study of gold redistribution in a shock-metamorphosed pyrite-quartz mixture with the use of the 195Au radionuclide [J]. Geochemistry International, 2004, 42(12): 1139–1153. [28] MOROSIN B, GRAHAM R A, POLLACK S S. X-ray diffraction line broadening in shock modified pyrite [M]//SCHMIDT S C, DICK R D, FORBES J W, et al. Shock Compression of Condensed Matter–1991. Amsterdam: North-Holland, 1992: 613−616. [29] POLLACK S S, MARTELLO D V, GRAHAM R A, et al. Reactivity of pyrites and dislocation density [M]//International Energy Agency Coal Research Ltd. 1991 International Conference on Coal Science Proceedings. Butterworth-Heinemann, 1991: 885−888. [30] SCLAR C B, USSELMAN T M. Experimentally induced shock effects in some rock-forming minerals [J]. Meteoritics, 1970, 5: 222–223. [31] AHRENS T J. Equations of state of iron sulfide and constraints on the sulfur content of the Earth [J]. Journal of Geophysical Research: Solid Earth, 1979, 84(B3): 985–998. doi: 10.1029/JB084iB03p00985 [32] AHRENS T J, JEANLOZ R. Pyrite: shock compression, isentropic release, and composition of the Earth’s core [J]. Journal of Geophysical Research: Solid Earth, 1987, 92(B10): 10363–10375. doi: 10.1029/JB092iB10p10363 [33] ANDERSON W W, AHRENS T J. Shock temperature and melting in iron sulfides at core pressures [J]. Journal of Geophysical Research: Solid Earth, 1996, 101(B3): 5627–5642. doi: 10.1029/95JB01972 [34] LANGENHORST F, BOUSTIE M, DEUTSCH A, et al. Experimental techniques for the simulation of shock metamorphism: a case study on calcite [M]//DAVISON L, HORIE Y, SEKINE T. High-Pressure Shock Compression of Solids V. New York: Springer, 2003: 1−27. [35] MARTINEZ I, DEUTSCH A, SCHÄRER U, et al. Shock recovery experiments on dolomite and thermodynamical calculations of impact induced decarbonation [J]. Journal of Geophysical Research: Solid Earth, 1995, 100(B8): 15465–15476. doi: 10.1029/95JB01151 [36] MANSFELD U, LANGENHORST F, EBERT M, et al. Microscopic evidence of stishovite generated in low-pressure shock experiments on porous sandstone: constraints on its genesis [J]. Meteoritics & Planetary Science, 2017, 52(7): 1449–1464. doi: 10.1111/maps.12867 [37] ZHANG F P, SEKINE T. Impact-shock behavior of Mg- and Ca-sulfates and their hydrates [J]. Geochimica et Cosmochimica Acta, 2007, 71(16): 4125–4133. doi: 10.1016/j.gca.2007.06.037 [38] KUROSAWA K, MORIWAKI R, KOMATSU G, et al. Shock vaporization/devolatilization of evaporitic minerals, halite and gypsum, in an open system investigated by a two-stage light gas gun [J]. Geophysical Research Letters, 2019, 46(13): 7258–7267. doi: 10.1029/2019GL083249 [39] PRESCHER C, LANGENHORST F, HORNEMANN U, et al. Shock experiments on anhydrite and new constraints on the impact-induced SO x release at the K-Pg boundary [J]. Meteoritics & Planetary Science, 2011, 46(11): 1619–1629. doi: 10.1111/j.1945-5100.2011.01249.x [40] GRIEVE R A F, LANGENHORST F, STÖFFLER D. Shock metamorphism of quartz in nature and experiment: Ⅱ. significance in geoscience [J]. Meteoritics & Planetary Science, 1996, 31(1): 6–35. doi: 10.1111/j.1945-5100.1996.tb02049.x [41] THOMPSON E C, CHIDESTER B A, FISCHER R A, et al. Equation of state of pyrite to 80 GPa and 2400 K [J]. American Mineralogist, 2016, 101(5): 1046–1051. doi: 10.2138/am-2016-5527 [42] ZHANG Y J, SEKINE T, LIN J F, et al. Shock compression and melting of an Fe-Ni-Si alloy: implications for the temperature profile of the Earth’s core and the heat flux across the core-mantle boundary [J]. Journal of Geophysical Research: Solid Earth, 2018, 123(2): 1314–1327. doi: 10.1002/2017JB014723 [43] KRAUS R G, DAVIS J P, SEAGLE C T, et al. Dynamic compression of copper to over 450 GPa: a high-pressure standard [J]. Physical Review B, 2016, 93(13): 134105. doi: 10.1103/PhysRevB.93.134105 [44] DAI C D, HU J B, TAN H. Hugoniot temperatures and melting of tantalum under shock compression determined by optical pyrometry [J]. Journal of Applied Physics, 2009, 106(4): 043519. doi: 10.1063/1.3204941 [45] MITCHELL A C, NELLIS W J. Shock compression of aluminum, copper, and tantalum [J]. Journal of Applied Physics, 1981, 52(5): 3363–3374. doi: 10.1063/1.329160 [46] SEILER F, IGRA O. Hypervelocity launchers [M]. Cham: Springer, 2016. [47] WANG H P, SALVESON I. A review on the mineral chemistry of the non-stoichiometric iron sulphide, Fe1− xS (0≤x≤0.125): polymorphs, phase relations and transitions, electronic and magnetic structures [J]. Phase Transitions, 2005, 78(7/8): 547–567. doi: 10.1080/01411590500185542 [48] GAN B, ZHANG Y J, HUANG Y Q, et al. Partial deoxygenation and dehydration of ferric oxyhydroxide in Earth’s subducting slabs [J]. Geophysical Research Letters, 2021, 48(17): e2021GL094446. doi: 10.1029/2021GL094446 [49] MANG C, KONTNY A, FRITZ J, et al. Shock experiments up to 30 GPa and their consequences on microstructures and magnetic properties in pyrrhotite [J]. Geochemistry, Geophysics, Geosystems, 2013, 14(1): 64–85. doi: 10.1029/2012GC004242 [50] LEROUX H, REIMOLD W U, KOEBERL C, et al. Experimental shock deformation in zircon: a transmission electron microscopic study [J]. Earth and Planetary Science Letters, 1999, 169(3/4): 291–301. doi: 10.1016/S0012-821X(99)00082-5 [51] RAO M N, NYQUIST L E, ASIMOW P D, et al. Shock experiments on basalt-ferric sulfate mixes and their possible relevance to the sulfide bleb clusters in large impact melts in shergottites [J]. Meteoritics & Planetary Science, 2021, 56(12): 2250–2264. doi: 10.1111/maps.13770 [52] RAO M N, NYQUIST L E, ROSS D K, et al. Signatures of the Martian regolith components entrained in some impact-melt glasses in shergottites [J]. Meteoritics & Planetary Science, 2018, 53(12): 2558–2582. doi: 10.1111/maps.13177 [53] DENG J S, WEN S M, CHEN X M, et al. Dynamic simulation of the thermal decomposition of pyrite under vacuum [J]. Metallurgical and Materials Transactions A, 2014, 45(5): 2445–2452. doi: 10.1007/s11661-014-2206-4 [54] LANGE M A, LAMBERT P, AHRENS T J. Shock effects on hydrous minerals and implications for carbonaceous meteorites [J]. Geochimica et Cosmochimica Acta, 1985, 49(8): 1715–1726. doi: 10.1016/0016-7037(85)90142-5 [55] MEYERS M A, ANDRADE U R, CHOKSHI A H. The effect of grain size on the high-strain, high-strain-rate behavior of copper [J]. Metallurgical and Materials Transactions A, 1995, 26(11): 2881–2893. doi: 10.1007/BF02669646 [56] SCHMITT R T. Shock experiments with the H6 chondrite Kernouvé: pressure calibration of microscopic shock effects [J]. Meteoritics & Planetary Science, 2000, 35(3): 545–560. doi: 10.1111/j.1945-5100.2000.tb01435.x [57] 苏林祥. 《实验物态方程导引》简介 [J]. 爆炸与冲击, 1987, 7(1): 84. doi: 10.11883/1001-1455(1987)01-0084-1SU L X. Introduction to the “equation of state for experimental states” [J]. Explosion and Shock Waves, 1987, 7(1): 84. doi: 10.11883/1001-1455(1987)01-0084-1 [58] GRAY Ⅲ G T. Shock recovery experiments: an assessment [C]//American Physical Society Topical Conference on Shock Compression of Condensed Matter. Albuquerque, 1989. [59] WILLIAMS C L, ZIMMERMAN K B. Structure-property relationships under extreme dynamic environments: shock recovery experiments [M]. Cham: Springer, 2019. [60] SEKINE T. Shock-induced chemistry [M]. Singapore: Springer, 2024. [61] RAIKES S A, AHRENS T J. Post-shock temperatures in minerals [J]. Geophysical Journal International, 1979, 58(3): 717–747. doi: 10.1111/j.1365-246X.1979.tb04804.x [62] GRAY Ⅲ G T, FOLLANSBEE P S, FRANTZ C E. Effect of residual strain on the substructure development and mechanical response of shock-loaded copper [J]. Materials Science and Engineering: A, 1989, 111: 9–16. doi: 10.1016/0921-5093(89)90192-5 [63] GRAY Ⅲ G T. Influence of shock-wave deformation on the structure/property behavior of materials [M]//ASAY J R, SHAHINPOOR M. High-Pressure Shock Compression of Solids. New York: Springer, 1993: 187−215. [64] HU J P, ASIMOW P D, MA C, et al. First synthesis of a unique icosahedral phase from the Khatyrka meteorite by shock-recovery experiment [J]. IUCrJ, 2020, 7(3): 434–444. doi: 10.1107/S2052252520002729 [65] BECKER L, POREDA R J, HUNT A G, et al. Impact event at the Permian-Triassic boundary: evidence from extraterrestrial noble gases in fullerenes [J]. Science, 2001, 291(5508): 1530–1533. doi: 10.1126/science.1057243 [66] BELL M S. CO2 release due to impact devolatilization of carbonate: results of shock experiments [J]. Meteoritics & Planetary Science, 2016, 51(4): 619–646. doi: 10.1111/maps.12613 [67] FRITZ J, GRESHAKE A, FERNANDES V A. Revising the shock classification of meteorites [J]. Meteoritics & Planetary Science, 2017, 52(6): 1216–1232. doi: 10.1111/maps.12845 [68] STÖFFLER D, KEIL K, SCOTT E R D. Shock metamorphism of ordinary chondrites [J]. Geochimica et Cosmochimica Acta, 1991, 55(12): 3845–3867. doi: 10.1016/0016-7037(91)90078-J [69] RUBIN A E. Smyer H-chondrite impact-melt breccia and evidence for sulfur vaporization [J]. Geochimica et Cosmochimica Acta, 2002, 66(4): 699–711. doi: 10.1016/S0016-7037(01)00799-2 [70] BENNETT III M E, MCSWEEN H Y. Shock features in iron-nickel metal and troilite of L-group ordinary chondrites [J]. Meteoritics & Planetary Science, 1996, 31(2): 255–264. doi: 10.1111/j.1945-5100.1996.tb02021.x [71] DAI C D, JIN X G, FU S Q, et al. The equation-of-states of Jilin ordinary chondrite and Nandan iron meteorite [J]. Science in China Series D: Earth Sciences, 1997, 40(4): 403–410. doi: 10.1007/BF02877572 [72] STÖFFLER D, BISCHOFF A, BUCHWALD V, et al. Shock effects in meteorites [M]. Meteorites and the Early Solar System, 1988. [73] HU J P, SHARP T G. Formation, preservation and extinction of high-pressure minerals in meteorites: temperature effects in shock metamorphism and shock classification [J]. Progress in Earth and Planetary Science, 2022, 9(1): 6. doi: 10.1186/s40645-021-00463-2 [74] IVANOV B A. Mars/moon cratering rate ratio estimates [J]. Space Science Reviews, 2001, 96(1/2/3/4): 87–104. doi: 10.1023/A:1011941121102 [75] FARINELLA P, DAVIS D R. Collision rates and impact velocities in the main asteroid belt [J]. Icarus, 1992, 97(1): 111–123. doi: 10.1016/0019-1035(92)90060-K [76] SAITO T, KAIHO K, ABE A, et al. Hypervelocity impact of asteroid/comet on the oceanic crust of the Earth [J]. International Journal of Impact Engineering, 2008, 35(12): 1770–1777. doi: 10.1016/j.ijimpeng.2008.07.046 [77] SAITO T, KAIHO K, ABE A, et al. Numerical simulations of hypervelocity impact of asteroid/comet on the Earth [J]. International Journal of Impact Engineering, 2006, 33(1): 713−722. [78] LI M H, FRANK T D, XU Y L, et al. Sulfur isotopes link atmospheric sulfate aerosols from the Siberian Traps outgassing to the end-Permian extinction on land [J]. Earth and Planetary Science Letters, 2022, 592: 117634. doi: 10.1016/j.jpgl.2022.117634 [79] KAIHO K, KAJIWARA Y, CHEN Z Q, et al. A sulfur isotope event at the end of the Permian [J]. Chemical Geology, 2006, 235(1/2): 33–47. doi: 10.1016/j.chemgeo.2006.06.001 [80] ERWIN D H, BOWRING S A, YUGAN J. End-Permian mass extinctions: a review [M]//KOEBERL C, MACLEOD K G. Catastrophic Events and Mass Extinctions: Impacts and Beyond. Boulder: Geological Society of America, 2002. [81] RUBIN A E. Maskelynite in asteroidal, lunar and planetary basaltic meteorites: an indicator of shock pressure during impact ejection from their parent bodies [J]. Icarus, 2015, 257: 221–229. doi: 10.1016/j.icarus.2015.05.010 [82] GENG S J, ZHOU B H, LI M T. On the capture of small stony asteroids into the Earth’s orbit by atmospheric grazing [J]. Monthly Notices of the Royal Astronomical Society, 2021, 507(3): 4661–4668. doi: 10.1093/mnras/stab2439 [83] MAHOWALD N, WARD D S, KLOSTER S, et al. Aerosol impacts on climate and biogeochemistry [J]. Annual Review of Environment and Resources, 2011, 36: 45–74. doi: 10.1146/annurev-environ-042009-094507 [84] FINLAYSON-PITTS B J, PITTS J N JR. Chemistry of the upper and lower atmosphere [M]. San Diego: Academic Press, 2000. [85] MACDONALD F A, WORDSWORTH R. Initiation of Snowball Earth with volcanic sulfur aerosol emissions [J]. Geophysical Research Letters, 2017, 44(4): 1938–1946. doi: 10.1002/2016GL072335 [86] STERN R J, AVIGAD D, MILLER N, et al. From volcanic winter to snowball Earth: an alternative explanation for neoproterozoic biosphere stress [M]//DILEK Y, FURNES H, MUEHLENBACHS K. Links Between Geological Processes, Microbial Activities & Evolution of Life: Microbes and Geology. Dordrecht: Springer, 2008: 313−337. [87] FAN S M, JACOB D J. Surface ozone depletion in Arctic spring sustained by bromine reactions on aerosols [J]. Nature, 1992, 359(6395): 522–524. doi: 10.1038/359522a0 [88] PORTMANN R W, SOLOMON S, GARCIA R R, et al. Role of aerosol variations in anthropogenic ozone depletion in the polar regions [J]. Journal of Geophysical Research: Atmospheres, 1996, 101(D17): 22991–23006. doi: 10.1029/96JD02608 [89] TABAZADEH A, DRDLA K, SCHOEBERL M R, et al. Arctic “ozone hole” in a cold volcanic stratosphere [J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(5): 2609–2612. doi: 10.1073/pnas.052518199 [90] BENCA J P, DUIJNSTEE I A P, LOOY C V. UV-B-induced forest sterility: implications of ozone shield failure in Earth’s largest extinction [J]. Science Advances, 2018, 4(2): e1700618. doi: 10.1126/sciadv.1700618 [91] VISSCHER H, LOOY C V, COLLINSON M E, et al. Environmental mutagenesis during the end-Permian ecological crisis [J]. Proceedings of the National Academy of Sciences of the United States of America, 2004, 101(35): 12952–12956. doi: 10.1073/pnas.0404472101 [92] BLACK B A, LAMARQUE J F, SHIELDS C A, et al. Acid rain and ozone depletion from pulsed Siberian Traps magmatism [J]. Geology, 2014, 42(1): 67–70. doi: 10.1130/G34875.1 [93] LI R C, SHEN S Z, XIA X P, et al. Atmospheric ozone destruction and the end-Permian crisis: evidence from multiple sulfur isotopes [J]. Chemical Geology, 2024, 647: 121936. doi: 10.1016/j.chemgeo.2024.121936 [94] JURIKOVA H, GUTJAHR M, WALLMANN K, et al. Permian-Triassic mass extinction pulses driven by major marine carbon cycle perturbations [J]. Nature Geoscience, 2020, 13(11): 745–750. doi: 10.1038/s41561-020-00646-4 -








 下载:
下载:






 下载:
下载: