Recent Progress on Structural and Functional Evolutions of Metal Halide Perovskites under High Pressure
-
摘要: 过去10年里,金属卤素钙钛矿作为一种性能优异的新型功能材料被广泛应用,其研究取得了很多重要进展。压力作为一个基本的热力学变量,可以显著地影响材料的微观结构、原子间相互作用、电子轨道和化学键,是调节材料结构和性能的一个强大工具。与此同时,压力也为研究结构与性质之间的关系提供了新路径。结合金刚石对顶砧高压装置以及原位高压表征技术,总结了金属卤素钙钛矿在高压下的结构及性质变化,包括高压驱动结构相变,有序-无序转变,非晶化,局部结构演化,带隙、光致发光、光响应、电阻等性质在压力作用下的变化,以及高压下特有的奇特性质如金属化转变,系统分析了此类材料的结构-性质关系,并对未来的新型材料设计做出了展望。Abstract: Over the past decade, metal halide perovskites have been widely employed as the emerging active-materials for technological innovations, and their research has become one of the central goals in the field of energetic materials. Pressure, a new thermodynamic dimension, can tune microstructure, atomic interactions, electronic orbitals, and chemical bonds of materials, thus serves as a potent means to regulate the structures and properties of metal halide perovskites. In addition, pressure paves a novel avenue for probing and understanding the structure-property relationship. Taking the advantage of diamond anvil cell technology and in situ high-pressure characterization techniques, we have comprehensively summarized the pressure-induced evolutions of metal halide perovskites, encompassing structural phase transitions, order-disorder transitions, amorphization, and local structural evolution. We have examined alterations in properties, such as bandgap, photoluminescence, photoelectronic response, and electrical resistance, and other distinctive high-pressure phenomena. This review systematically analyzes the structure-property interplay within these known materials, and offers insights into the design of future novel materials.
-
Key words:
- high-pressure /
- metal halide perovskite /
- structural evolution /
- semiconductor /
- diamond anvil cell
-
年轮式超高压装置自发明以来,已被广泛应用于金刚石和立方氮化硼等超硬材料合成,满足了切割、钻孔、研磨等加工工业领域对超硬材料的需求[1–2]。年轮式超高压装置主要由年轮式超高压模具和2个对称布置的顶锤组成,其中,年轮式超高压模具由硬质合金压缸和多层高强合金钢支撑环组成[3–4]。模具的腔体内为合成物质,并采用叶蜡石或MgO作为传压介质进行密封,利用液压机驱动2个对称布置的顶锤对腔体内物质进行挤压来产生超高压力环境,该模具可稳定产生5~10 GPa的压力[5]。年轮式超高压装置具有压力梯度和温度梯度稳定、样品腔体积大的优点,也被广泛用作科学研究装置,如凝聚态物理、高压化学和地球科学等领域[6–7]。
年轮式超高压装置的设计工作主要集中在提高腔体的工作尺寸和极限承压能力2个方面。提高这2项指标的优势主要表现在:工业合成金刚石和立方氮化硼时,可显著增加产量,降低能耗,并促进合成物质的稳定生长,使产品品质得到明显提升;可为现代测试技术提供更加稳定的测试环境,更精准地获得测量物质的理化性质数据,并增加数据采集量;可为功能材料的设计合成、高压条件下测试物质的原子结构和新理化性质提供更大腔体、更高压力环境。
在实际设计过程中,腔体的工作尺寸和极限承压能力2项指标是一对矛盾的存在。增大腔体体积,其承压能力会降低;为了获得较高的承压能力,需要减小模具腔体体积。同时,硬质合金压缸工作时,压缸内壁会产生非常高的周向拉应力和剪切应力,从而限制装置的极限承压能力和使用寿命,影响合成物质的产品品质,且增加装置的使用成本[8–9]。大尺寸的腔体必然对应大尺寸的硬质合金压缸,受硬质合金生产工艺所限,大尺寸硬质合金加工十分困难,并且成本高昂[10]。因此,年轮式超高压模具腔体大型化受到大尺寸硬质合金加工难和腔体压力难以提高的限制。
为了维持腔体内压力的稳定,需要采用多层支撑环对剖分式压缸进行预紧,导致支撑环的尺寸增加。此外,大尺寸高强钢支撑环的加工也存在诸多困难,如腔体体积为500 cm3的年轮式模具,其支撑环的尺寸可达1.2 m,加工难度极大,特别是热处理质量不均匀容易导致支撑环在装配和工作时断裂[11]。目前,钢丝或钢带缠绕预紧方法已被应用于大型压机设计领域,但尚未用于合成人造金刚石的超高压模具设计中。
为了提高年轮式模具的极限承压能力、降低支撑环尺寸,进而实现腔体大型化,基于大质量支撑原理和侧向支撑原理,研究人员提出了一种钢丝缠绕离散式超高压模具,即将压缸进行离散,只保留一层支撑环对离散后的压缸进行固定和提前预紧,支撑环外部为钢丝缠绕层,进而实现对离散式压缸的充分预紧。压缸离散后,其周向应力得到有效降低,在外部支撑环与钢丝缠绕层预紧的作用下,压缸离散块相互挤压,提供了更好的大质量和侧向支撑效果,可有效提高模具极限承压能力。同时,硬质合金零件尺寸大大减小,离散后的硬质合金体积仅为整体式压缸的十几分之一,降低了硬质合金的制造难度[12]。本研究将对缠绕离散式大腔体超高压模具进行设计计算,通过有限元方法对模具应力状态进行分析,并预测其极限承压能力,以期为大腔体、高承压能力的超高压模具设计提供新思路、新方法。
1. 模具的受力分析及计算
模具的受力分析及计算满足以下基本假设:(1) 根据缠绕离散式超高压模具的工作环境,假设钢丝层及各层均处于弹性范围;(2) 钢丝层缠绕紧密,工作内压引起的应力在整个缠绕层厚度上是连续的,且工作压力可通过Lamé公式[13]计算;(3) 忽略模具端部对应力和变形的影响,作为平面问题处理。
钢丝满足以下基本要求:(1) 钢丝截面均匀,无误差或公差较小;(2) 钢丝组织无缺陷、表面较光滑,且塑性较高;(3) 钢丝韧性较高,工作过程中不会发生断裂。
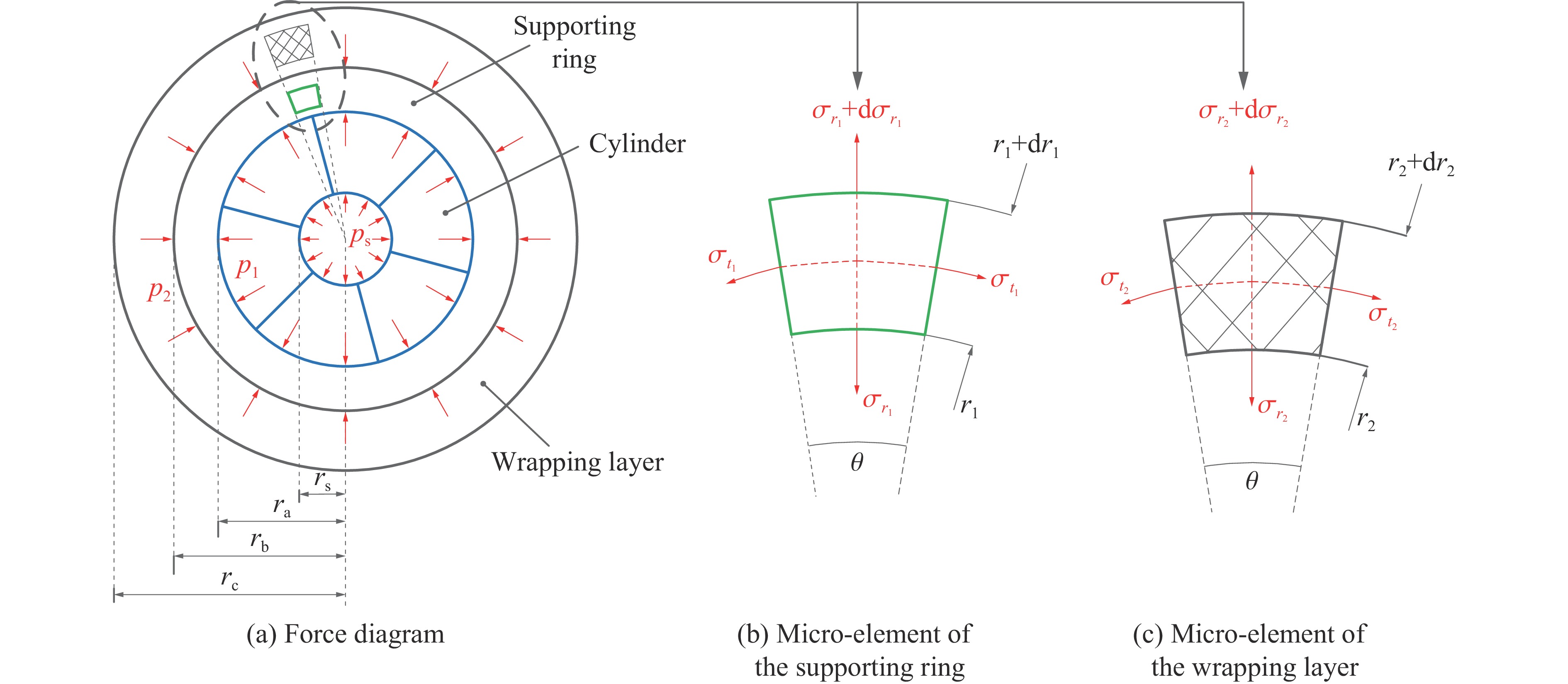
缠绕离散式大腔体超高压模具受力如图1所示,其中:rs、ra分别为压缸内径、外径,rb为支撑环外径,rc为钢丝缠绕层外径,θ为微元角度。对于离散式压缸,设腔体高度为h,压缸高度为H,有
psrsh=p1raH (1) 式中:ps为工作内压,p1为压缸与支撑环之间的压力。由式(1)可得,p1=psrsh/raH。对于支撑环内部,受离散块传递的内压和钢丝预紧力的作用,在支撑环内r1处产生周向应力σt1和径向应力σr1,根据微元平衡条件可得
σt1−σr1=r1dσr1dr1 (2) 支撑环上周向和径向的剪应力为
τ1=σt1−σr12 (3) 通过式(2)、式(3)可得支撑环ra和rb处的径向应力σra和σrb的关系为
σra−σrb=2∫rbraτ1r1dr1 (4) 通过边界条件可知,支撑环的内壁压力p1=−σra,外壁压力p2=−σrb,进一步可得
p1−p2=2∫rbraτ1dlnr1 (5) 支撑环材料的剪切强度为τ∗1,对式(5)进行积分可得
p1−p2=2τ∗1lnrbra=2τ∗1lnK=τ∗1lnK2 (6) 式中:K为支撑环外径与压缸外径之比。
对于钢丝缠绕层内任意r2处,同样取一个微元体,且微元体受力平衡与支撑环微元体受力平衡计算方法一致,有
σt2−σr2=r2dσr2dr2 (7) 钢丝以等张力F缠绕的方式进行缠绕预紧,任意半径r2(rb≤r2≤rc)处受到外层钢丝对其预紧产生的径向应力为σr2,切向应力为σ′t2,根据Lamé公式有
σ′t2=r22+r2ar22−r2aσr2 (8) 而钢丝层内r2处微元的切向应力σt2应为钢丝张力F与钢丝层内切向应力σ′t2的叠加,即
σt2=F−σ′t2=F−r22+r2ar22−r2aσr2 (9) 联立式(8)和式(9),得到
F+(1−r22+r2ar22−r2a)σr2+r2dσr2dr2=0 (10) 由模具的边界条件可知,当r2=rc时,σr2=0,可得到张力为F的缠绕层半径r2处的径向预紧力
σr2=F(r22−r2a2r22)lnr2c−r2ar22−r2a (11) 而支撑环外半径处的径向预紧力为σrb,通过式(11)可求得
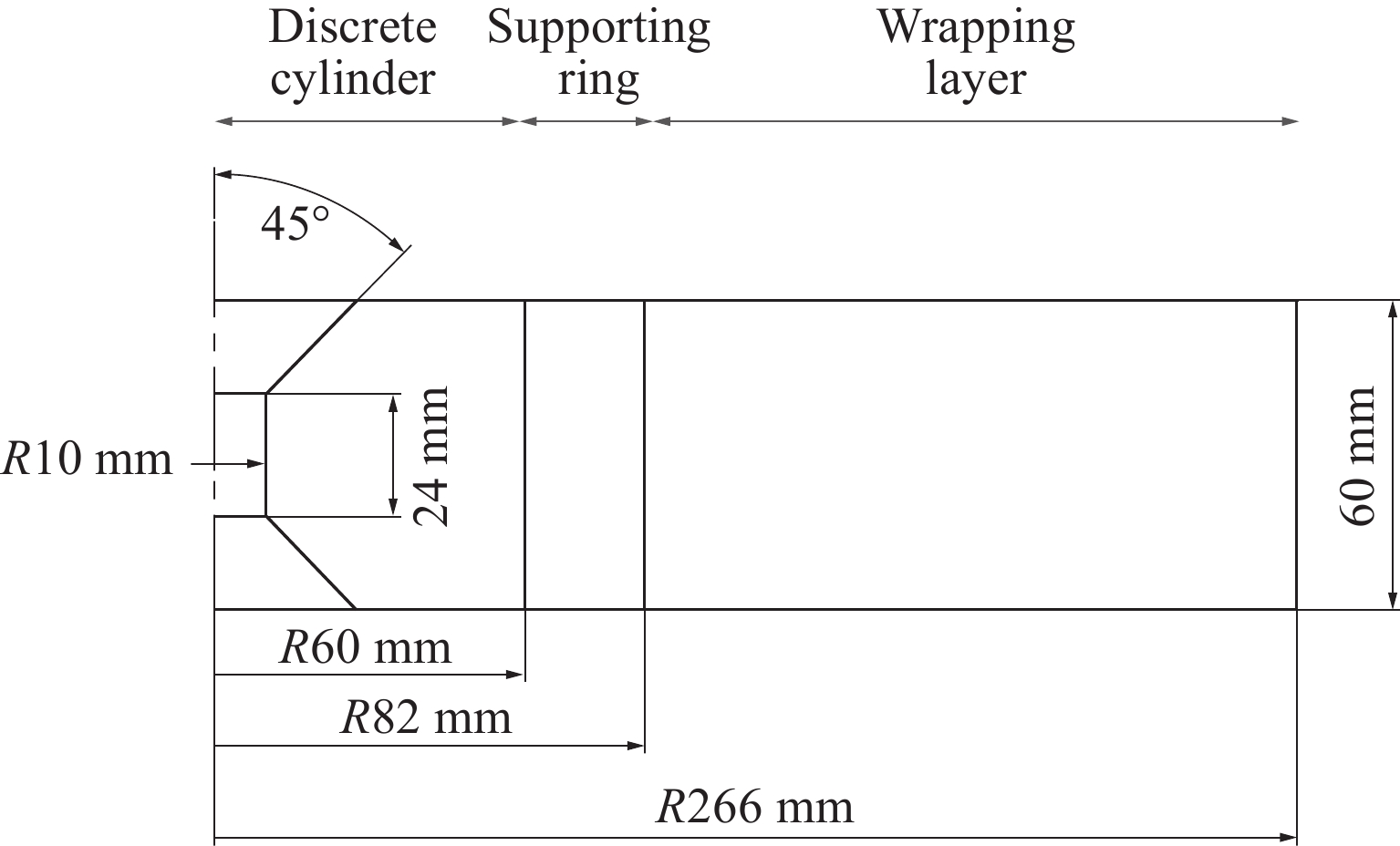
r2c=(r2b−r2a)exp2r2bσrb(r2b−r2a)F+r2a (12) 压缸材料为硬质合金YG8,支撑环材料为优质合金钢45CrNiMoVA,钢丝材料为65Mn。考虑到模具的使用工况,压缸、支撑环和钢丝材料的安全系数分别取1.0、1.1和1.1,具体参数如表1所示。本研究中硬质合金压缸腔体半径为10 mm,压缸外半径为60 mm。设定腔体内的均布压力为6 GPa,支撑环外圈径向预紧力为500 MPa,钢丝缠绕张力为700 MPa。计算的模具尺寸如图2所示。
2. 有限元分析
2.1 有限元模型
采用数值模拟获得模具的受力情况并进行对比,综合模具腔体体积,得到模具腔体高度、模具高度等参数的最优值。压缸腔体高为24 mm,模具高为60 mm,压缸锥面角度为45°,压缸腔体的工作压力p0为6 GPa,压缸锥面的正压力p(s)和密封介质对锥面的摩擦力f(s)与腔体内的工作压力存在以下关系
p(s)=p0e−2τsδ (13) f(s)=f0p0e−2τsδ (14) 式中:τ为传压介质的内摩擦系数,δ为压缸锥面上密封介质的厚度,s为密封层任意一点到压缸边缘的距离,f0为密封介质与压缸之间的摩擦系数。
模具的缠绕层对支撑环和离散式压缸的预紧可通过在支撑环外施加一个等效的预紧外压来模拟,对离散成60°、45°、36°和30°的离散式压缸进行分析。为了缩短计算时间,分别对缠绕离散式超高压模具建立1/12、1/16、1/20和1/24有限元模型。有限元的网格尺寸为1.5 mm,类型为Solid186。采用有限元方法分析模具的受力状态,并预测模具的极限承压能力,同时与整体式压缸进行对比。
2.2 模具应力分析与讨论
2.2.1 模具应力分析
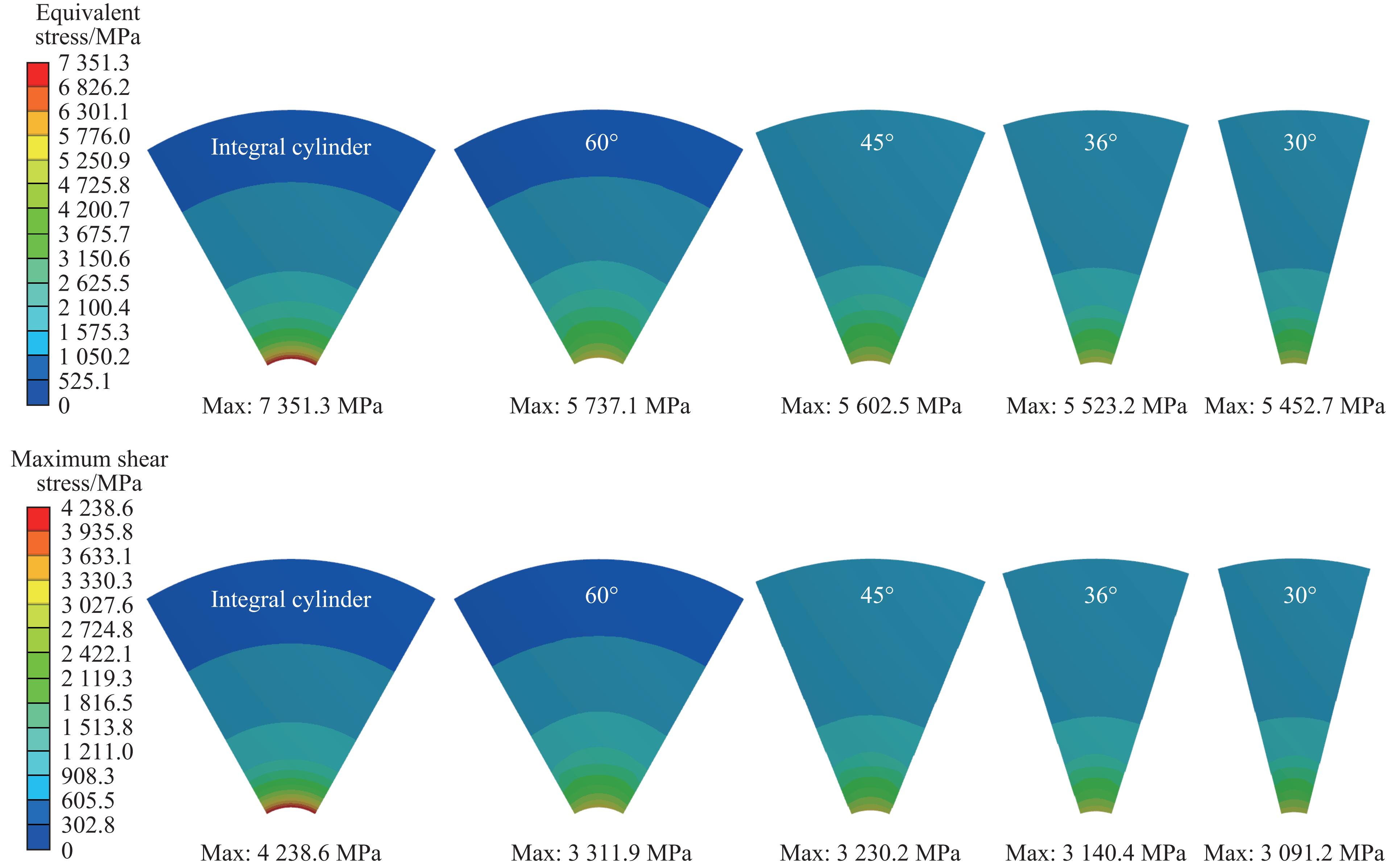
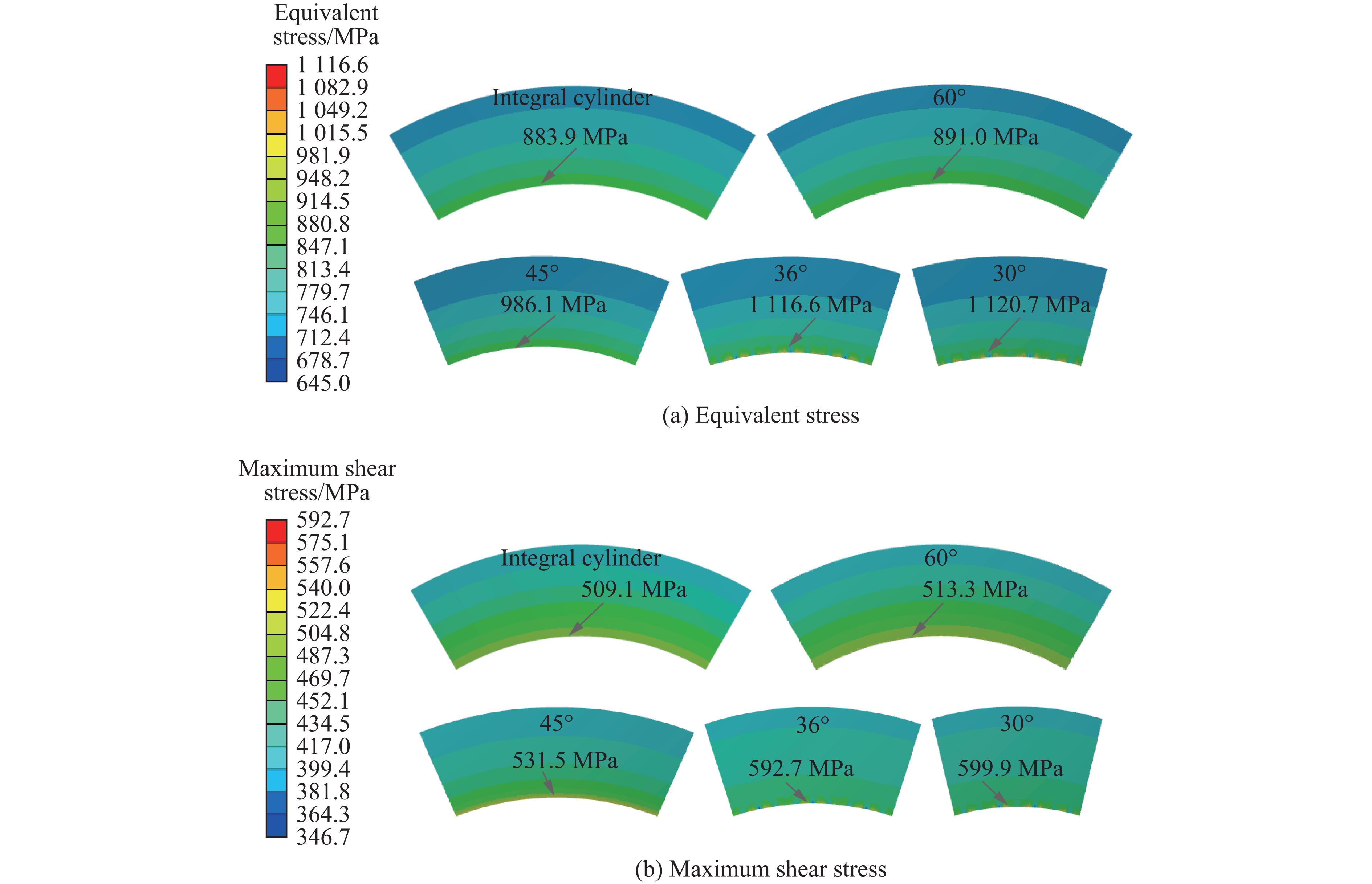
模具工作时,腔体内产生的超高压力会在整体式压缸内壁产生极大的周向拉应力,而硬质合金类脆性材料承受拉应力的能力较低,限制了模具的极限承压能力。整体式压缸经过离散化处理,腔体的工作压力主要沿着模具的径向传递,又由于钢丝缠绕预紧的作用,使得离散式压缸主要受压应力的作用。因此,压缸受力情况得到明显改善。压缸应力随不同离散角度的变化情况如图3所示。
整体式压缸的等效应力和最大切应力分别为7 351.3和4 238.6 MPa。采用最大畸变能理论和最大剪切理论作为判断模具失效的依据,由表1可见,硬质合金压缸在此工况下已经被破坏。压缸内壁与外壁的应力差距较大,这是由于压缸的工作内压在压缸内壁产生了较大的周向应力,而压缸外壁由于受到预紧的压应力作用,导致硬质合金材料没有得到最大化利用。可以看到:离散成60°的压缸已经遭到破坏,而其余离散式压缸可继续提高压力并正常工作;随着压缸离散块离散角度的减小,应力逐渐降低。与整体式压缸相比,离散式压缸的等效应力和最大切应力平均值分别降低了24.1%和24.7%。
基于以上的结果和分析可以推断,离散式压缸可承受更高的压力,而支撑环由于受到外部钢丝缠绕预紧作用,可有效保证模具的安全使用。硬质合金离散块的体积只有整体式压缸的几分之一甚至十几分之一,有效避免了大尺寸硬质合金零件的使用,同时破损的压缸离散块也易于更换。因此,离散式压缸的结构优势可显著降低模具的制造难度和工业应用成本,也能够为超硬材料的生产和科学研究提供大腔体、超高压力的物理环境。
2.2.2 压缸内壁应力分析
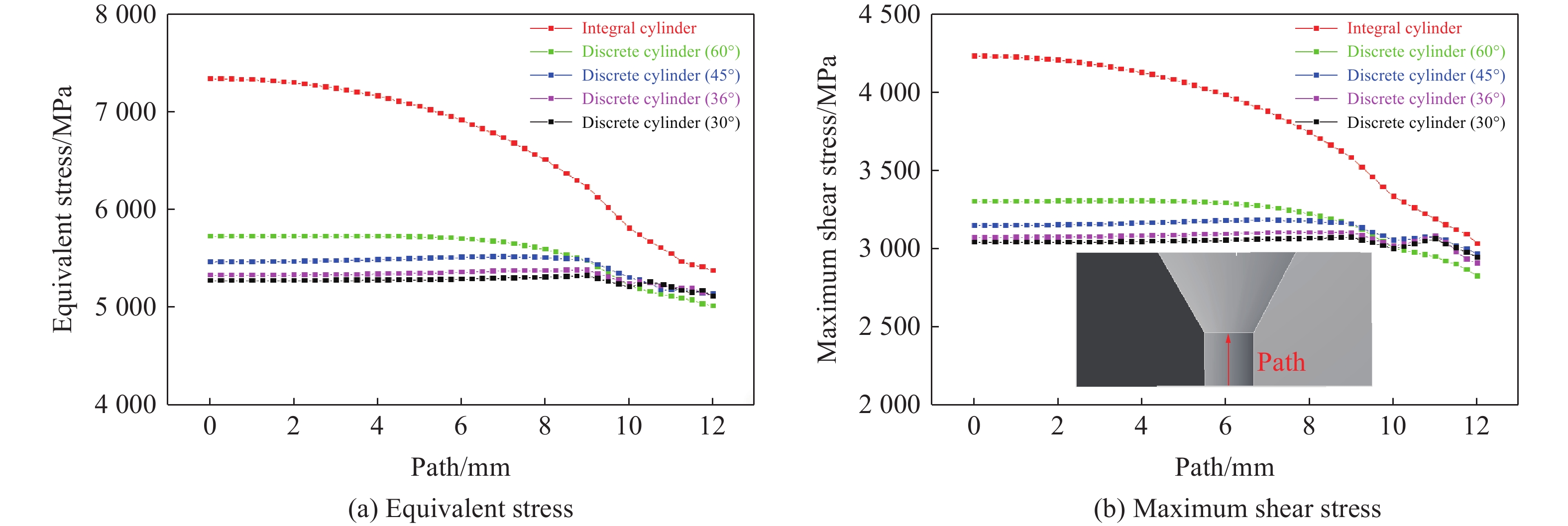
硬质合金压缸内壁是承受压力的主要区域,其应力状态对模具的极限承压能力存在重要影响。为方便分析,统计了如图4所示路径上的应力。结果显示,在腔体内部加载压力相等的情况下,与整体式压缸相比,离散式压缸应力明显降低。对于整体式压缸,压缸中部的等效应力和最大剪切应力较大,而端部的应力分别为5 477.1和3 150.0 MPa,腔体中部的等效应力和最大剪切应力比腔体端部分别高1 968和1 411 MPa,压缸中部与端部的应力差值较大,压缸受力不均匀,应力集中明显,限制了压缸的极限承压能力。与整体式压缸相比,离散式压缸内壁应力明显降低,并且这2种应力沿路径的分布较为平缓,应力分布较为均匀。压缸中部的应力随着离散化的进行逐渐降低,但是降低的趋势越来越缓慢,说明不能通过无限增加离散块数量来降低压缸内壁应力。离散式压缸内壁的受力情况得到明显改善,应力沿路径的分布较为平缓,可以推断,离散式压缸可承受更高的压力。
2.2.3 支撑环应力分析
缠绕离散式大腔体超高压模具的支撑环内壁对离散式压缸进行提前预紧,外壁受到钢丝缠绕层的预紧力作用,其应力状态也会对模具的使用产生重要影响。图5给出了支撑环的等效应力和最大剪切应力分布情况。从图5可以看出,沿着径向,等效应力和最大剪切应力均逐渐降低,最大值出现在支撑环内壁的中间部位,且年轮式支撑环内壁在周向上的应力分布均匀。由于离散式压缸在周向上是不连续的,使得其支撑环内壁的等效应力和最大剪切应力在周向上同样不连续,并呈现周期性分布。
在同样的加载条件下,随着压缸离散块数量的增加,支撑环的应力逐渐增大,与整体式压缸相比,离散式压缸的支撑环等效应力分别增加了0.8%、11.5%、26.3%和26.7%,最大剪切应力分别增加了0.8%、4.4%、16.4%和17.8%。支撑环应力虽然也在逐渐增大,但是增大的趋势越来越慢。需要特别注意的是,支撑环的应力全部在使用范围内。通过对支撑环内壁应力进行分析可知,随着压缸离散块数量的增加,压缸内壁逐渐出现应力集中趋势,且离散块数量越多,应力集中趋势越明显。这是因为,离散块外壁与支撑环内壁接触面之间在预紧力与工作内压的共同作用下,产生了较大的摩擦力,离散块数量越多,摩擦力在周向上的分布越不均匀,使其表现为应力集中趋势。
2.3 剖分式压缸承压能力预测
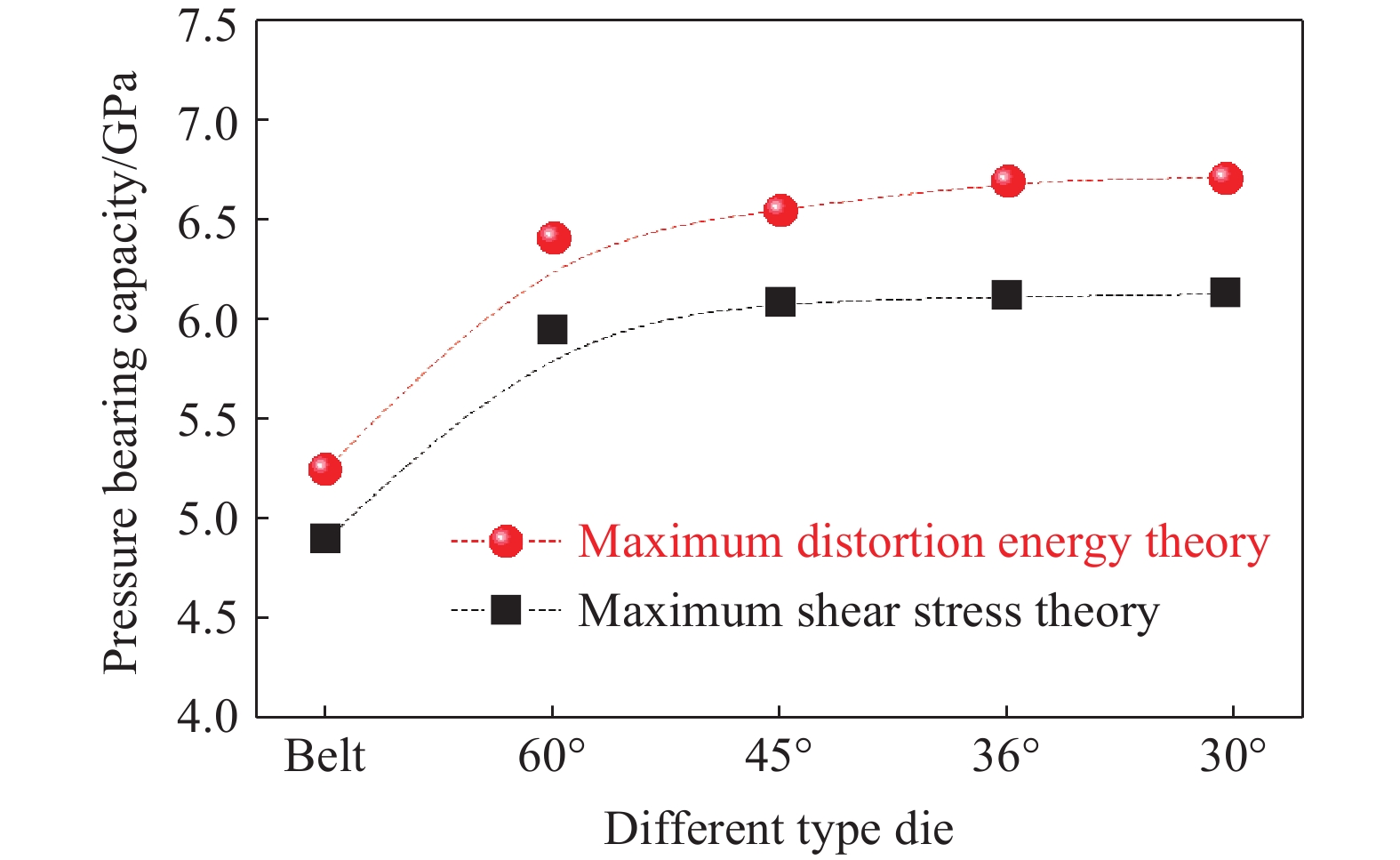
利用有限元法对缠绕离散式大腔体超高压模具的极限承压能力进行预测,在有限元模型中,以压缸腔体内的压力为设计变量,范围为4.0~7.5 GPa,每间隔0.1 GPa作为一个设计点,以等效应力和最大剪切应力作为输出参数,并以最大畸变能理论和最大剪切应力理论作为失效判据,当应力达到压缸和支撑环的强度极限时,模具失效。图6给出了预测的模具极限承压能力的变化趋势。依据2个失效判据,整体式压缸的极限承压能力分别为5.31和4.93 GPa。根据最大畸变能理论,60°、45°、36°和30°离散式压缸的极限承压能力分别为6.41、6.55、6.70和6.75 GPa;当采用最大剪切应力理论时,承压能力分别为5.95、6.10、6.15和6.21 GPa。离散式压缸的承压能力明显优于年轮整体式压缸,平均增加了24.3%和23.7%。
从图6还可以看出,压缸离散块数量越多,承压能力越强,但是增加的趋势越来越缓慢。硬质合金的加工成本与加工面数量相关,离散块数量增多会导致制造成本增加。因此,结合压缸的应力分析可知,通过无限增加离散式压缸离散块数量来增加极限承压能力是不可行的。总体来说,离散式压缸无需使用大尺寸硬质合金,可充分保证硬质合金零件的烧结质量;并且压缸遭到破坏后,更换破损的压缸即可重新使用,降低了模具的维护和使用成本。
3. 结 论
提出了一种缠绕离散式大腔体超高压模具结构,即将整体式压缸进行离散化处理,形成组合式压缸结构,通过一层支撑环和钢丝缠绕层进行有效预紧,使压缸具有优异的大质量支撑和侧向支撑效果,其极限承压能力更强,无需使用大尺寸硬质合金,显著降低了模具腔体的大型化难度。分析了模具各层的应力,推导了关键结构参数的计算公式,并采用数值模拟方法获得了模具的应力状态和应力特征并进行分析。与整体式压缸相比,离散式压缸的极限承压能力更强,并且维护和使用成本更低,更易于实现腔体大型化。
对模具极限承压能力与离散块数量关系的研究发现,通过无限提高压缸离散块数量来提高承压能力是不可取的。因此,压缸离散数量的选取原则可以归纳为:当模具压缸尺寸较大时,压缸离散块的数量应该更多,以降低硬质合金制造难度并保障压缸的极限承压能力;当模具的压缸体积较小时,离散块数量应该更少,以降低加工制造费用,同时也能够保证模具的承压能力。
-
图 2 MHPVs中压力驱动的非晶化、有序-无序转变、局部无序:(a) MASnI3在压缩和再压缩时压力驱动的结构演化[25],(b) Cs2AgBiBr6在2.1 GPa时隐藏的局部无序[36]
Figure 2. Pressure-driven amorphous, ordered-disordered transition, local disorder: (a) pressure-driven structural evolution of MASnI3 during compression and recompression in MHPVs[25]; (b) Cs2AgBiBr6 local disorder at 2.1 GPa[36]
图 3 高压实验条件及时间对结构和性能的影响:(a)不同传压介质(pressure-transmitting-medium,PTM)下MAPbBr3在同一压力时的结构[15];高压下MAPbCl3的结构(b)和性能(c)的时间依赖[14]
Figure 3. Effect of high-pressure experimental conditions and time on structure and performance: (a) the structure of MAPbBr3 at the same pressure under different pressure-transmitting-media[15]; time dependence of structure (b) and performance (c) of MAPbCl3 under high pressure[14]
图 4 (a)~(b) 结合中红外峰证明压力下的有序-无序转变[69],其中,FWHM为半峰宽,d为晶面间距,d0为常压下的晶面间距
Figure 4. (a)−(b) order-disordered transitions under pressure demonstrated by FWHM and d/d0 and mid-infrared peaks,where FWHM represent full width at half maximum, d represents the interplanar spacing under the current pressure, and d0 represents the interplanar spacing under ambient condition[69]
图 5 带隙的压力依赖性:(a) 不同卤素钙钛矿中的带隙演化[11, 26, 31, 41–42, 55, 63, 69, 76, 85, 89, 97, 105, 107],(b) 压缩下Pb―I―Pb键的键长和键角的变化[68]
Figure 5. Pressure dependence of band gap:(a) band gap evolution in different halogen perovskites[11, 26, 31, 41–42, 55, 63, 69, 76, 85, 89, 97, 105, 107]; (b) changes of bond length and bond angle of Pb―I―Pb bonds under compression[68]
图 6 不同MHPVs PL的压力依赖:(a) MAPbI1.2Br1.8[9]的PL谱,(b) (2meptH2)PbCl4钙钛矿的PL压力依赖和压力下的光学图像[43],(c) Cs2AgBiCl6[96]的PL谱,(d)~(e)常温常压和高压下自捕获激子发射的演化示意图[96],(f) CsPbBr3单晶的PL压力依赖[106]
Figure 6. Pressure dependence of different MHPVs PL: (a) MAPbI1.2Br1.8[9]; (b) pressure dependence of PL and optical image of (2meptH2)PbCl4[43]; (c) Cs2AgBiCl6[96]; (d)–(e) schematic diagram of the evolution of self-captured exciton emission under environmental conditions and high pressure[38]; (f) pressure dependence of PL in CsPbBr3 single crystal[106]
图 7 (a) MAPbI3的压力依赖PL衰减动力学[11],(b) MAPbI3靠近价带顶的缺陷态在压力下变浅[11],(c) MAPbI3的弱间接带隙[120],(d) α-FAPbI3的压力依赖PL衰减动力学[26],(e) 不同压力下MAPbI3 MC的压力依赖PL衰减动力学[19],(f) MAPbI3 MC平均PL寿命和PL强度的压力依赖[19],(g) CsPbBr3 NCs载流子寿命和带隙的压力依赖[29]
Figure 7. (a) MAPbI3 pressure-dependent PL attenuation kinetics[11]; (b) pressure dependence of defect states in MAPbI3[11]; (c) weak indirect bandgap of MAPbI3[120]; (d) α-FAPbI3 pressure-dependent PL attenuation kinetics[26]; (e) pressure-dependent PL decay kinetics of MAPbI3 MC[19]; (f) pressure dependence of average PL lifetime and PL intensity of MAPbI3 MC[19]; (g) pressure dependence of carrier lifetime and bandgap in CsPbBr3 NCs[29]
图 8 压力驱动的不同MHPVs的光电性质的演化:(a)~(b) MAPbBr3[23],(c)~(d) MASnI3[25],(e)~(g) MAPbBr3多晶的电输运性能[126],(h)~(i) Cs3Bi2I9的增强的光电流和宽带光响应[65],(j) Cs2PbI2Cl2在2 GPa下的显著的光电流增强[44],(k)压力促进的激子解离示意图[44]
Figure 8. Pressure-driven evolution of photoelectric properties of different MHPVs: (a)–(b) MAPbBr3[23]; (c)–(d) MASnI3[25];(e)–(g) electrical transport performance of MAPbBr3 polycrystalline [126]; (h)–(i) enhanced photocurrent and broadband light response of Cs3Bi2I9[65]; (j) significant photocurrent enhancement at 2 GPa for Cs2PbI2Cl2[44]; (k) schematic diagram of stress-facilitated exciton dissociation[44]
图 9 高压下MHPVs的金属化:(a)~(b) MAPbI3带隙的压力依赖[13],(c) 高压下MAPbI3的红外反射光谱[13],(d) 高压下MAPbI3的电导率的温度依赖[13],(e) Cs2In(Ⅰ)In(Ⅲ)Cl6高压原位拉曼光谱[127],(f) Cs2In(I)In(III)Cl6的压力依赖光吸收光谱[127],(g)~(h) 压力下CD3ND3PbI3的红外吸收光谱[18],(i) 72 GPa压力下CD3ND3PbI3的带隙为零[18]
Figure 9. MHPVs metallization under high pressure: (a)–(b) pressure dependence of the MAPbI3 bandgap[13]; (c) infrared reflectance spectrum of MAPbI3 at high pressure[13]; (d) temperature dependence of MAPbI3 conductivity at high pressure[13]; (e) high-pressure in situ Raman of Cs2In(Ⅰ)In(Ⅲ )Cl6[127]; (f) pressure-dependent light absorption spectra of Cs2In(Ⅰ)In(Ⅲ )Cl6[127];(g)–(h) CD3ND3PbI3 IR absorption spectra under pressure[18]; (i) CD3ND3PbI3 with zero bandgap at 72 GPa[18]
图 10 不同MHPVs的压致发光行为:(a)~(c) Cs4PbBr6 NCs从3.01 GPa开始表现出明显的光发射[119],(d) Cs4PbBr6 NCs的与压力相关的色度坐标[119],(e)~(f) (BA)4AgBiBr8在高压下的PL光谱[39],(g) (BA)4AgBiBr8的PL位置和PL强度的压力依赖[39],(h) (BA)4AgBiBr8在高压下的光学图像(PL随压力的增加而变化)[39],(i)~(j) C4N2H14SnBr4的压力依赖PL光谱和与压力相关的色度坐标[114]
Figure 10. Pressed luminescence of different MHPVS:(a)–(c) Cs4PbBr6 nanocrystals begin to exhibit significant emission at high pressure of 3.01GPa[119]; (d) pressure-dependent chromaticity coordinates of Cs4PbBr6 nanocrystals[119] ; (e)–(f) PL spectroscopy of (BA)4AgBiBr8 at high pressure[39]; (g) pressure dependence of PL position and PL strength of (BA)4AgBiBr8[39]; (h) the optical pattern of (BA)4AgBiBr8 at high pressure shows that PL varies with increasing pressure[39]; (i)–(j) pressure dependent PL spectrum and pressure-dependent chromaticity coordinates of C4N2H14SnBr4[114]
表 1 金属卤化物钙钛矿的压力诱导相变
Table 1. Pressure-induced phase transitions of metal halide perovskite
Material Phase transitions Ref. MAPbBr3 Pm¯3m (ambient pressure)→Im¯3 (0.4 GPa)→Pnma (1.8 GPa) [23] MAPbI3 I4/mcm (ambient pressure)→Imm2 (0.26 GPa) [8] MAPbCl3 Pm¯3m (ambient pressure)→Pm¯3m (0.8 GPa)→Pnma (2.0 GPa) [12] CD3ND3PbI3 I4/mcm (ambient pressure)→Imm2 (1.30 GPa)→Imm (2.57 GPa) [18] MASnI3 P4mm (ambient pressure)→Pnma (0.7 GPa) [25] MAPbI1.2Br1.8 Pm¯3m (ambient pressure)→Im¯3 (2.7 GPa) [9] MASnCl3 Pc (ambient pressure)→P1 (1 GPa)→amorphization (above 3 GPa) [24] MA3Bi2Br9 P¯3m1 (ambient pressure)→P21/a (5 GPa) [64] FAPbBr3 Pm¯3m (ambient pressure)→Im¯3 (0.53 GPa)→Pnma (2.2 GPa) [90] FAPbI3 No phase transitions below 7 GPa [26] FAPbI3 NCs Pm¯3m (ambient pressure)→Im¯3(0.6 GPa) [27] α-FAPbI3 Pm¯3m→Imm2 (0.3 GPa), Imm2→Immm (1.7 GPa) [28] (C9NH20)6Pb3Br12 No phase transitions below 80 GPa [38] DABCuCl4 P21/a (ambient pressure)→P2 (6.4 GPa) [63] BA2PbI4 Pbca (ambient pressure)→P21/a (2 GPa) [22] MHy2PbBr4 Pmn21→P21 (near 4 GPa) [74] Cy4BiBr7 No phase transitions below 20.13 GPa [84] CsPbBr3 Isostructural phase transition (about 1.2 GPa) [29] CsPbBr3 Isostructural phase transition (1.2 GPa) [98] CsPbBr3 Pbnm (ambient pressure)→Pm3m (1.7 GPa) [33] RP-CsPbBr3 Pbnm (ambient pressure)→P21/m (0.74 GPa ) [33] CsPbI3 Pnma (ambient pressure)→P21/m (5.6 GPa) [30] Cs2SnBr6 No phase transitions below 20 GPa [34] Cs2AgBiBr6 Fm¯3m (ambient pressure)→I4/m (4.5 GPa) [99] Cs3Bi2I9 No phase transitions below 12.7 GPa [97] Cs3Bi2I9 No phase transitions below 20.3 GPa [93] Cs3Bi2Br9 P¯3m1 (ambient pressure)→C2/c (10.1 GPa) [37] Cs2AgBiCl6 Fm¯3m (ambient pressure)→I4/m (5.6 GPa) [96] Cs2PbI2Cl2 I4/mmm (ambient pressure)→C2/m (2.8 GPa) [44] 表 2 不同MHPVs的PL发生和消失压力
Table 2. Pressures corresponding PL occurrence and disappearance for different MHPVs
Material Dimension Initial pressure of PL/GPa PL annihilation pressure/GPa Ref. MAPbCl3 3D Ambient 7.20 [108] MAPbBr3 3D Ambient 4.85 [108] MAPbBr3 3D Ambient 4.00 [109] MAPbI3 3D Ambient 2.70 [10] MAPbI1.2Br1.8 3D Ambient 1.60 [9] CsPb2Br5 3D Ambient 2.23 [40] CsPbBr3 3D Ambient 2.40 [106] Cs2AgBiCl6 3D Ambient 8.00 [96] (BA)2PbI4 2D Ambient 10.00 [110] (BA)2PbI4 2D Ambient 12.60 [22] (PEA)2PbBr4 2D Ambient 15.60 [41] (PEA)2PbI4 2D Ambient 7.60 [111] (HA)2(GA)Pb2I7 2D Ambient 9.48 [112] (BA)2(MA)Pb2I7 2D Ambient 4.70 [69] (GA)(MA)2Pb2I7 2D Ambient 7.00 [46] (BA)4AgBiBr8 2D 2.50 25.00 [39] C4N2H14PbBr4 1D Ambient 9.00 [91] C4N2H14PbBr4 1D Ambient 24.81 [113] C4N2H14SnBr4 1D 2.06 20.02 [114] CH3(CH2)2NH3PbBr3 1D Ambient 7.30 [115] CsCu2I3 1D Ambient 16.00 [116] (bmpy)9[ZnBr4]2[Pb3Br11] 0D Ambient 18.20 [117] (bmpy)6[Pb3Br12] 0D Ambient >80 [38] (MA)3Bi2I9 0D Ambient 9.00 [118] Cs4PbBr6 0D 3.01 18.23 [119] Cs3Bi2I9 0D Ambient 9.30 [97] -
[1] TRAVIS W, GLOVER E N K, BRONSTEIN H, et al. On the application of the tolerance factor to inorganic and hybrid halide perovskites: a revised system [J]. Chemical Science, 2016, 7(7): 4548–4556. doi: 10.1039/C5SC04845A [2] KIESLICH G, SUN S J, CHEETHAM A K. An extended tolerance factor approach for organic-inorganic perovskites [J]. Chemical Science, 2015, 6(6): 3430–3433. doi: 10.1039/C5SC00961H [3] BOYD C C, CHEACHAROEN R, LEIJTENS T, et al. Understanding degradation mechanisms and improving stability of perovskite photovoltaics [J]. Chemical Reviews, 2019, 119(5): 3418–3451. doi: 10.1021/acs.chemrev.8b00336 [4] Best research-cell efficiency chart [EB/OL]. [2023-10-15]. https://www.nrel.gov/pv/cell-efficiency.html. [5] CHEN X Y, XIE J J, WANG W, et al. Research progress of compositional controlling strategy to perovskite for high performance solar cells [J]. Acta Chimica Sinica, 2019, 77(1): 9–23. doi: 10.6023/a18100447 [6] LIU G, KONG L P, YANG W G, et al. Pressure engineering of photovoltaic perovskites [J]. Materials Today, 2019, 27: 91–106. doi: 10.1016/j.mattod.2019.02.016 [7] KOJIMA A, TESHIMA K, SHIRAI Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells [J]. Journal of the American Chemical Society, 2009, 131(17): 6050–6051. doi: 10.1021/ja809598r [8] CAPITANI F, MARINI C, CARAMAZZA S, et al. High-pressure behavior of methylammonium lead iodide (MAPbI3) hybrid perovskite [J]. Journal of Applied Physics, 2016, 119(18): 185901. doi: 10.1063/1.4948577 [9] JAFFE A, LIN Y, BEAVERS C M, et al. High-pressure single-crystal structures of 3D lead-halide hybrid perovskites and pressure effects on their electronic and optical properties [J]. ACS Central Science, 2016, 2(4): 201–209. doi: 10.1021/acscentsci.6b00055 [10] JIANG S J, FANG Y N, LI R P, et al. Pressure-dependent polymorphism and band-gap tuning of methylammonium lead iodide perovskite [J]. Angewandte Chemie International Edition, 2016, 55(22): 6540–6544. doi: 10.1002/anie.201601788 [11] KONG L P, LIU G, GONG J, et al. Simultaneous band-gap narrowing and carrier-lifetime prolongation of organic-inorganic trihalide perovskites [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(32): 8910–8915. doi: 10.1073/pnas.1609030113 [12] WANG L R, WANG K, XIAO G J, et al. Pressure-induced structural evolution and band gap shifts of organometal halide perovskite-based methylammonium lead chloride [J]. The Journal of Physical Chemistry Letters, 2016, 7(24): 5273–5279. doi: 10.1021/acs.jpclett.6b02420 [13] JAFFE A, LIN Y, MAO W L, et al. Pressure-induced metallization of the halide perovskite (CH3NH3)PbI3 [J]. Journal of the American Chemical Society, 2017, 139(12): 4330–4333. doi: 10.1021/jacs.7b01162 [14] SZAFRAŃSKI M, KATRUSIAK A. Photovoltaic hybrid perovskites under pressure [J]. The Journal of Physical Chemistry Letters, 2017, 8(11): 2496–2506. doi: 10.1021/acs.jpclett.7b00520 [15] ZHANG R, CAI W Z, BI T G, et al. Effects of nonhydrostatic stress on structural and optoelectronic properties of methylammonium lead bromide perovskite [J]. The Journal of Physical Chemistry Letters, 2017, 8(15): 3457–3465. doi: 10.1021/acs.jpclett.7b01367 [16] JIANG H Y, XUE H T, WANG L F, et al. Effect of pressure-induced structural phase transition on electronic and optical properties of perovskite CH3NH3PbI3 [J]. Materials Science in Semiconductor Processing, 2019, 96: 59–65. doi: 10.1016/j.mssp.2019.01.038 [17] LEE J H, JAFFE A, LIN Y, et al. Origins of the pressure-induced phase transition and metallization in the halide perovskite (CH3NH3)PbI3 [J]. ACS Energy Letters, 2020, 5(7): 2174–2181. doi: 10.1021/acsenergylett.0c00772 [18] KONG L P, GONG J, HU Q Y, et al. Suppressed lattice disorder for large emission enhancement and structural robustness in hybrid lead iodide perovskite discovered by high-pressure isotope effect [J]. Advanced Functional Materials, 2021, 31(9): 2009131. doi: 10.1002/adfm.202009131 [19] YIN Y F, TIAN W M, LUO H, et al. Excellent carrier transport property of hybrid perovskites sustained under high pressures [J]. ACS Energy Letters, 2022, 7(1): 154–161. doi: 10.1021/acsenergylett.1c02359 [20] JUNG Y K, ABDULLA M, FRIEND R H, et al. Pressure-induced non-radiative losses in halide perovskite light-emitting diodes [J]. Journal of Materials Chemistry C, 2022, 10(35): 12560–12568. doi: 10.1039/D2TC01490D [21] OU T J, YAN J J, XIAO C H, et al. Visible light response, electrical transport, and amorphization in compressed organolead iodine perovskites [J]. Nanoscale, 2016, 8(22): 11426–11431. doi: 10.1039/C5NR07842C [22] YUAN Y, LIU X F, MA X D, et al. Large band gap narrowing and prolonged carrier lifetime of (C4H9NH3)2PbI4 under High Pressure [J]. Advanced Science, 2019, 6(15): 1900240. doi: 10.1002/advs.201900240 [23] WANG Y G, LYU X J, YANG W G, et al. Pressure-induced phase transformation, reversible amorphization, and anomalous visible light response in organolead bromide perovskite [J]. Journal of the American Chemical Society, 2015, 137(34): 11144–11149. doi: 10.1021/jacs.5b06346 [24] WANG L R, OU T J, WANG K, et al. Pressure-induced structural evolution, optical and electronic transitions of nontoxic organometal halide perovskite-based methylammonium tin chloride [J]. Applied Physics Letters, 2017, 111(23): 233901. doi: 10.1063/1.5004186 [25] LÜ X J, WANG Y G, STOUMPOS C C, et al. Enhanced structural stability and photo responsiveness of CH3NH3SnI3 perovskite via pressure-induced amorphization and recrystallization [J]. Advanced Materials, 2016, 28(39): 8663–8668. doi: 10.1002/adma.201600771 [26] LIU G, KONG L P, GONG J, et al. Pressure-induced bandgap optimization in lead-based perovskites with prolonged carrier lifetime and ambient retainability [J]. Advanced Functional Materials, 2017, 27(3): 1604208. doi: 10.1002/adfm.201604208 [27] ZHU H, CAI T, QUE M D, et al. Pressure-induced phase transformation and band-gap engineering of formamidinium lead iodide perovskite nanocrystals [J]. The Journal of Physical Chemistry Letters, 2018, 9(15): 4199–4205. doi: 10.1021/acs.jpclett.8b01852 [28] WANG P, GUAN J W, GALESCHUK D T K, et al. Pressure-induced polymorphic, optical, and electronic transitions of formamidinium lead iodide perovskite [J]. The Journal of Physical Chemistry Letters, 2017, 8(10): 2119–2125. doi: 10.1021/acs.jpclett.7b00665 [29] XIAO G J, CAO Y, QI G Y, et al. Pressure effects on structure and optical properties in cesium lead bromide perovskite nanocrystals [J]. Journal of the American Chemical Society, 2017, 139(29): 10087–10094. doi: 10.1021/jacs.7b05260 [30] YUAN G, QIN S, WU X, et al. Pressure-induced phase transformation of CsPbI3 by X-ray diffraction and Raman spectroscopy [J]. Phase Transitions, 2018, 91(1): 38–47. doi: 10.1080/01411594.2017.1357180 [31] CAO Y, QI G Y, LIU C, et al. Pressure-tailored band gap engineering and structure evolution of cubic cesium lead iodide perovskite nanocrystals [J]. The Journal of Physical Chemistry C, 2018, 122(17): 9332–9338. doi: 10.1021/acs.jpcc.8b01673 [32] LIANG Y F, HUANG X L, HUANG Y P, et al. New metallic ordered phase of perovskite CsPbI3 under pressure [J]. Advanced Science, 2019, 6(14): 1900399. doi: 10.1002/advs.201900399 [33] YESUDHAS S, MORRELL M V, ANDERSON M J, et al. Pressure-induced phase changes in cesium lead bromide perovskite nanocrystals with and without Ruddlesden-Popper faults [J]. Chemistry of Materials, 2020, 32(2): 785–794. doi: 10.1021/acs.chemmater.9b04157 [34] YUAN G, HUANG S X, NIU J J, et al. Compressibility of Cs2SnBr6 by X-ray diffraction and Raman spectroscopy [J]. Solid State Communications, 2018, 275: 68–72. doi: 10.1016/j.ssc.2018.03.014 [35] FU R J, CHEN Y P, YONG X, et al. Pressure-induced structural transition and band gap evolution of double perovskite Cs2AgBiBr6 nanocrystals [J]. Nanoscale, 2019, 11(36): 17004–17009. doi: 10.1039/C9NR07030C [36] GIRDZIS S P, LIN Y, LEPPERT L, et al. Revealing local disorder in a silver-bismuth halide perovskite upon compression [J]. The Journal of Physical Chemistry Letters, 2021, 12(1): 532–536. doi: 10.1021/acs.jpclett.0c03412 [37] GENG T, WEI S, ZHAO W Y, et al. Insight into the structure-property relationship of two-dimensional lead-free halide perovskite Cs3Bi2Br9 nanocrystals under pressure [J]. Inorganic Chemistry Frontiers, 2021, 8(6): 1410–1415. doi: 10.1039/D0QI01300E [38] FANG Y Y, ZHANG L, WU L W, et al. Pressure-induced emission (PIE) and phase transition of a two-dimensional halide double perovskite (BA)4AgBiBr8 (BA = CH3(CH2)3NH3+) [J]. Angewandte Chemie International Edition, 2019, 58(43): 15249–15253. doi: 10.1063/5.0058821 [39] CHEN M T, GUO S H, BU K J, et al. Pressure-induced robust emission in a zero-dimensional hybrid metal halide (C9NH20)6Pb3Br12 [J]. Matter and Radiation at Extremes, 2021, 6(5): 08401. doi: 10.1002/anie.201906311 [40] MA Z W, LI F F, QI G Y, et al. Structural stability and optical properties of two-dimensional perovskite-like CsPb2Br5 microplates in response to pressure [J]. Nanoscale, 2019, 11(3): 820–825. doi: 10.1039/C8NR05684F [41] ZHANG L, WU L W, WANG K, et al. Pressure-induced broadband emission of 2D organic-inorganic hybrid perovskite (C6H5C2H4NH3)2PbBr4 [J]. Advanced Science, 2019, 6(2): 1801628. doi: 10.1002/advs.201801628 [42] ZHAN X H, JIANG X M, LV P, et al. Enhanced structural stability and pressure-induced photoconductivity in two-dimensional hybrid perovskite (C6H5CH2NH3)2 CuBr4 [J]. Angewandte Chemie International Edition, 2022, 61(28): e202205491. doi: 10.1002/anie.202205491 [43] FANG Y Y, WANG J T, ZHANG L, et al. Tailoring the high-brightness “warm” white light emission of two-dimensional perovskite crystals via a pressure-inhibited nonradiative transition [J]. Chemical Science, 2023, 14(10): 2652–2658. doi: 10.1039/D2SC06982B [44] GUO S H, BU K J, LI J W, et al. Enhanced photocurrent of all-inorganic two-dimensional perovskite Cs2PbI2Cl2 via pressure-regulated excitonic features [J]. Journal of the American Chemical Society, 2021, 143(6): 2545–2551. doi: 10.1021/jacs.0c11730 [45] AZEEM M, QIN Y, LI Z G, et al. Cooperative B-site octahedral tilting, distortion and A-site conformational change induced phase transitions of a 2D lead halide perovskite [J]. Materials Chemistry Frontiers, 2021, 5(20): 7587–7594. doi: 10.1039/D1QM00566A [46] CHEN Y P, FU R J, WANG L R, et al. Emission enhancement and bandgap retention of a two-dimensional mixed cation lead halide perovskite under high pressure [J]. Journal of Materials Chemistry A, 2019, 7(11): 6357–6362. doi: 10.1039/C8TA11992A [47] CODURI M, STROBEL T A, SZAFRANSKI M, et al. Band gap engineering in MASnBr3 and CsSnBr3 perovskites: mechanistic insights through the application of pressure [J]. The Journal of Physical Chemistry Letters, 2019, 10(23): 7398–7405. doi: 10.1021/acs.jpclett.9b03046 [48] COHEN B E, WIERZBOWSKA M, ETGAR L. High efficiency and high open circuit voltage in quasi 2D perovskite based solar cells [J]. Advanced Functional Materials, 2017, 27(5): 1604733. doi: 10.1002/adfm.201604733 [49] FU R J, CHEN Y P, WANG L R, et al. Stability and band gap engineering of silica-confined lead halide perovskite nanocrystals under high pressure [J]. Geoscience Frontiers, 2021, 12(2): 957–963. doi: 10.1016/j.gsf.2020.07.004 [50] FU R J, ZHAO W Y, WANG L R, et al. Pressure-induced emission toward harvesting cold white light from warm white light [J]. Angewandte Chemie International Edition, 2021, 60(18): 10082–10088. doi: 10.1002/anie.202015395 [51] GAO F F, LI X, QIN Y, et al. Dual-stimuli-responsive photoluminescence of enantiomeric two-dimensional lead halide perovskites [J]. Advanced Optical Materials, 2021, 9(23): 2100003. doi: 10.1002/adom.202100003 [52] GAO F F, SONG H P, LI Z G, et al. Pressure-tuned multicolor emission of 2D lead halide perovskites with ultrahigh color purity [J]. Angewandte Chemie International Edition, 2023, 62(12): e202218675. doi: 10.1002/anie.202218675 [53] GAO X J, WANG Q, ZHANG Y, et al. Pressure effects on optoelectronic properties of CsPbBr3 nanocrystals [J]. The Journal of Physical Chemistry C, 2020, 124(20): 11239–11247. doi: 10.1021/acs.jpcc.0c02701 [54] GENG T, MA Z W, CHEN Y P, et al. Bandgap engineering in two-dimensional halide perovskite Cs3Sb2I9 nanocrystals under pressure [J]. Nanoscale, 2020, 12(3): 1425–1431. doi: 10.1039/C9NR09533K [55] GENG T, SHI Y, LIU Z, et al. Pressure-induced emission from all-inorganic two-dimensional vacancy-ordered lead-free metal halide perovskite nanocrystals [J]. The Journal of Physical Chemistry Letters, 2022, 13(50): 11837–11843. doi: 10.1021/acs.jpclett.2c03332 [56] GHOSH D, AZIZ A, DAWSON J A, et al. Putting the squeeze on lead iodide perovskites: pressure-induced effects to tune their structural and optoelectronic behavior [J]. Chemistry of Materials, 2019, 31(11): 4063–4071. doi: 10.1021/acs.chemmater.9b00648 [57] GHOSH P S, PONOMAREVA I. Negative linear compressibility in organic-inorganic hybrid perovskite [NH2NH3]X(HCOO)3 (X = Mn, Fe, Co) [J]. The Journal of Physical Chemistry Letters, 2022, 13(13): 3143–3149. doi: 10.1021/acs.jpclett.2c00288 [58] HUANG J S, YUAN Y B, SHAO Y C, et al. Understanding the physical properties of hybrid perovskites for photovoltaic applications [J]. Nature Reviews Materials, 2017, 2(7): 17042. doi: 10.1038/natrevmats.2017.42 [59] KE F, WANG C X, JIA C J, et al. Preserving a robust CsPbI3 perovskite phase via pressure-directed octahedral tilt [J]. Nature Communications, 2021, 12(1): 461. doi: 10.1038/s41467-020-20745-5 [60] KHOLIL M I, BHUIYAN M T H. Effects of pressure on narrowing the band gap, visible light absorption, and semi-metallic transition of lead-free perovskite CsSnBr3 for optoelectronic applications [J]. Journal of Physics and Chemistry of Solids, 2021, 154: 110083. doi: 10.1016/j.jpcs.2021.110083 [61] LI H, QIN Y, SHAN B H, et al. Unusual pressure-driven phase transformation and band renormalization in 2D vdW hybrid lead halide perovskites [J]. Advanced Materials, 2020, 32(12): 1907364. doi: 10.1002/adma.201907364 [62] LI H, WINES D, CHEN B, et al. Abnormal phase transition and band renormalization of guanidinium-based organic-inorganic hybrid perovskite [J]. ACS Applied Materials & Interfaces, 2021, 13(37): 44964–44971. doi: 10.1021/acsami.1c14521 [63] LI Q, LI S R, WANG K, et al. High-pressure study of perovskite-like organometal halide: band-gap narrowing and structural evolution of [NH3-(CH2)4-NH3]CuCl4 [J]. The Journal of Physical Chemistry Letters, 2017, 8(2): 500–506. doi: 10.1021/acs.jpclett.6b02786 [64] LI Q, YIN L X, CHEN Z W, et al. High pressure structural and optical properties of two-dimensional hybrid halide perovskite (CH3NH3)3Bi2Br9 [J]. Inorganic Chemistry, 2019, 58(2): 1621–1626. doi: 10.1021/acs.inorgchem.8b03190 [65] LI Z L, JIA B X, FANG S X, et al. Pressure-tuning photothermal synergy to optimize the photoelectronic properties in amorphous halide perovskite Cs3Bi2I9 [J]. Advanced Science, 2023, 10(6): 2205837. doi: 10.1002/advs.202205837 [66] LIANG Y F, WU M, TIAN C, et al. Pressure-tuned quantum well configuration in two-dimensional PA8Pb5I18 perovskites for highly efficient yellow fluorescence [J]. ACS Applied Energy Materials, 2021, 4(9): 10003–10011. doi: 10.1021/acsaem.1c01925 [67] LIANG Y F, ZANG Y F, HUANG X L, et al. Broadband emission enhancement induced by self-trapped excited states in one-dimensional EAPbI3 perovskite under pressure [J]. The Journal of Physical Chemistry C, 2020, 124(16): 8984–8991. doi: 10.1021/acs.jpcc.0c01553 [68] LIU G, GONG J, KONG L P, et al. Isothermal pressure-derived metastable states in 2D hybrid perovskites showing enduring bandgap narrowing [J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(32): 8076–8081. doi: 10.1073/pnas.1809167115 [69] LIU G, KONG L P, GUO P J, et al. Two regimes of bandgap red shift and partial ambient retention in pressure-treated two-dimensional perovskites [J]. ACS Energy Letters, 2017, 2(11): 2518–2524. doi: 10.1021/acsenergylett.7b00807 [70] LLOYD A J, HESTER B R, BAXTER S J, et al. Hybrid double perovskite containing helium: [He2][CaZr]F6 [J]. Chemistry of Materials, 2021, 33(9): 3132–3138. doi: 10.1021/acs.chemmater.0c04782 [71] LÜ X J, YANG W G, JIA Q X, et al. Pressure-induced dramatic changes in organic-inorganic halide perovskites [J]. Chemical Science, 2017, 8(10): 6764–6776. doi: 10.1039/C7SC01845B [72] MA Y L, ZHANG L, TANG Y, et al. Pressure-induced piezochromism and structure transitions in lead-free layered Cs4MnBi2Cl12 quadruple perovskite [J]. ACS Applied Energy Materials, 2021, 4(8): 7513–7518. doi: 10.1021/acsaem.1c01583 [73] MA Z W, LI Q, LUO J J, et al. Pressure-driven reverse intersystem crossing: new path toward bright deep-blue emission of lead-free halide double perovskites [J]. Journal of the American Chemical Society, 2021, 143(37): 15176–15184. doi: 10.1021/jacs.1c06207 [74] MĄCZKA M, SOBCZAK S, RATAJCZYK P, et al. Pressure-driven phase transition in two-dimensional perovskite MHy2PbBr4 [J]. Chemistry of Materials, 2022, 34(17): 7867–7877. doi: 10.1021/acs.chemmater.2c01533 [75] NICHOLAS A D, ZHAO J, SLEBODNICK C, et al. High-pressure structural and optical property evolution of a hybrid indium halide perovskite [J]. Journal of Solid State Chemistry, 2021, 300: 122262. doi: 10.1016/j.jssc.2021.122262 [76] RATTÉ J, MACINTOSH M F, DILORETO L, et al. Spacer-dependent and pressure-tuned structures and optoelectronic properties of 2D hybrid halide perovskites [J]. The Journal of Physical Chemistry Letters, 2023, 14(2): 403–412. doi: 10.1021/acs.jpclett.2c03555 [77] SAEED M, ALI M A, MURAD S, et al. Pressure induced structural, electronic, optical and thermal properties of CsYbBr3, a theoretical investigation [J]. Journal of Materials Research and Technology, 2021, 10: 687–696. doi: 10.1016/j.jmrt.2020.12.052 [78] SAMANTA D, SAHA P, GHOSH B, et al. Pressure-induced emergence of visible luminescence in lead free halide perovskite Cs3Bi2Br9: effect of structural distortion [J]. The Journal of Physical Chemistry C, 2021, 125(6): 3432–3440. doi: 10.1021/acs.jpcc.0c10624 [79] SHEN P F, VOGT T, LEE Y. Pressure-induced enhancement of broad-band white light emission in butylammonium lead bromide [J]. The Journal of Physical Chemistry Letters, 2020, 11(10): 4131–4137. doi: 10.1021/acs.jpclett.0c01160 [80] SHERWOOD B, RIDLEY C J, BULL C L, et al. A pressure induced reversal to the 9R perovskite in Ba3MoNbO8.5 [J]. Journal of Materials Chemistry A, 2021, 9(10): 6567–6574. doi: 10.1039/D0TA11270D [81] SHI Y, JIN Z Q, LV P F, et al. Bandgap narrowing and piezochromism of doped two-dimensional hybrid perovskite nanocrystals under pressure [J]. Journal of Materials Chemistry C, 2023, 11(5): 1726–1732. doi: 10.1039/D2TC05158C [82] SHI Y, ZHAO W Y, MA Z W, et al. Self-trapped exciton emission and piezochromism in conventional 3D lead bromide perovskite nanocrystals under high pressure [J]. Chemical Science, 2021, 12(44): 14711–14717. doi: 10.1039/D1SC04987A [83] SONG C P, YANG H R, LIU F, et al. Ultrafast femtosecond pressure modulation of structure and exciton kinetics in 2D halide perovskites for enhanced light response and stability [J]. Nature Communications, 2021, 12(1): 4879. doi: 10.1038/s41467-021-25140-2 [84] SUN M E, GENG T, YONG X, et al. Pressure-triggered blue emission of zero-dimensional organic bismuth bromide perovskite [J]. Advanced Science, 2021, 8(9): 2004853. doi: 10.1002/advs.202004853 [85] SUN M E, WANG Y G, WANG F, et al. Chirality-dependent structural transformation in chiral 2D perovskites under high pressure [J]. Journal of the American Chemical Society, 2023, 145(16): 8908–8916. doi: 10.1021/jacs.2c12527 [86] SUN S J, DENG Z Y, WU Y, et al. Variable temperature and high-pressure crystal chemistry of perovskite formamidinium lead iodide: a single crystal X-ray diffraction and computational study [J]. Chemical Communications, 2017, 53(54): 7537–7540. doi: 10.1039/C7CC00995J [87] SZAFRAŃSKI M, KATRUSIAK A, STÅHL K. Time-dependent transformation routes of perovskites CsPbBr3 and CsPbCl3 under high pressure [J]. Journal of Materials Chemistry A, 2021, 9(17): 10769–10779. doi: 10.1039/D1TA01875B [88] TIAN C, LIANG Y F, CHEN W H, et al. Hydrogen-bond enhancement triggered structural evolution and band gap engineering of hybrid perovskite (C6H5CH2NH3)2PbI4 under high pressure [J]. Physical Chemistry Chemical Physics, 2020, 22(4): 1841–1846. doi: 10.1039/C9CP05904K [89] WANG J X, WANG L R, WANG F, et al. Pressure-induced bandgap engineering of lead-free halide double perovskite (NH4)2SnBr6 [J]. Physical Chemistry Chemical Physics, 2021, 23(35): 19308–19312. doi: 10.1039/D1CP03267D [90] WANG L R, WANG K, ZOU B. Pressure-induced structural and optical properties of organometal halide perovskite-based formamidinium lead bromide [J]. The Journal of Physical Chemistry Letters, 2016, 7(13): 2556–2562. doi: 10.1021/acs.jpclett.6b00999 [91] WANG Y Q, GUO S H, LUO H, et al. Reaching 90% photoluminescence quantum yield in one-dimensional metal halide C4N2H14PbBr4 by pressure-suppressed nonradiative loss [J]. Journal of the American Chemical Society, 2020, 142(37): 16001–16006. doi: 10.1021/jacs.0c07166 [92] WANG Y J, ZHANG L K, MA S L, et al. Octahedral tilting dominated phase transition in compressed double perovskite Ba2SmBiO6 [J]. Applied Physics Letters, 2021, 118(23): 231903. doi: 10.1063/5.0054742 [93] WU L W, DONG Z Y, ZHANG L, et al. High-pressure band-gap engineering and metallization in the perovskite derivative Cs3Sb2I9 [J]. ChemSusChem, 2019, 12(17): 3971–3976. doi: 10.1002/cssc.201901388 [94] XIANG G B, WU Y W, ZHANG M, et al. Dimension-dependent bandgap narrowing and metallization in lead-free halide perovskite Cs3Bi2X9 (X = I, Br, and Cl) under high pressure [J]. Nanomaterials, 2021, 11(10): 2712. doi: 10.3390/nano11102712 [95] YANG H J, SHI W W, NAGAOKA Y, et al. Access and capture of layered double perovskite polytypic phase through high-pressure engineering [J]. The Journal of Physical Chemistry C, 2023, 127(5): 2407–2415. doi: 10.1021/acs.jpcc.2c07970 [96] ZHANG L, FANG Y Y, SUI L Z, et al. Tuning emission and electron-phonon coupling in lead-free halide double perovskite Cs2AgBiCl6 under pressure [J]. ACS Energy Letters, 2019, 4(12): 2975–2982. doi: 10.1021/acsenergylett.9b02155 [97] ZHANG L, LIU C M, WANG L R, et al. Pressure-induced emission enhancement, band-gap narrowing, and metallization of halide perovskite Cs3Bi2I9 [J]. Angewan Chemie International Edition, 2018, 57(35): 11213–11217. doi: 10.1002/anie.201804310 [98] ZHANG L, ZENG Q X, WANG K. Pressure-induced structural and optical properties of inorganic halide perovskite CsPbBr3 [J]. The Journal of Physical Chemistry Letters, 2017, 8(16): 3752–3758. doi: 10.1021/acs.jpclett.7b01577 [99] LI Q, WANG Y G, PAN W C, et al. High-pressure band-gap engineering in lead-free Cs2AgBiBr6 double perovskite [J]. Angewandte Chemie International Edition, 2017, 56(50): 15969–15973. doi: 10.1002/anie.201708684 [100] SHARMA S M, SIKKA S K. Pressure induced amorphization of materials [J]. Progress in Materials Science, 1996, 40(1): 1–77. doi: 10.1016/0079-6425(95)00006-2 [101] SHOCKLEY W, QUEISSER H J. Detailed balance limit of efficiency of p-n junction solar cells [J]. Journal of Applied Physics, 1961, 32(3): 510–519. doi: 10.1063/1.1736034 [102] LI M, LIU T B, WANG Y G, et al. Pressure responses of halide perovskites with various compositions, dimensionalities, and morphologies [J]. Matter and Radiation at Extremes, 2020, 5(1): 018201. doi: 10.1063/1.5133653 [103] ZHAO W J, RIBEIRO R M, EDA G. Electronic structure and optical signatures of semiconducting transition metal dichalcogenide nanosheets [J]. Accounts of Chemical Research, 2015, 48(1): 91–99. doi: 10.1021/ar500303m [104] TAUC J, GRIGOROVICI R, VANCU A. Optical properties and electronic structure of amorphous germanium [J]. Physica Status Solidi (b), 1966, 15(2): 627–637. doi: 10.1002/pssb.19660150224 [105] GAO C F, LI R P, LI Y R, et al. Direct-indirect transition of pressurized two-dimensional halide perovskite: role of benzene ring stack ordering [J]. The Journal of Physical Chemistry Letters, 2019, 10(19): 5687–5693. doi: 10.1021/acs.jpclett.9b02604 [106] GONG J B, ZHONG H X, GAO C, et al. Pressure-induced indirect-direct bandgap transition of CsPbBr3 single crystal and its effect on photoluminescence quantum yield [J]. Advanced Science, 2022, 9(29): 2201554. doi: 10.1002/advs.202201554 [107] WANG J X, WANG L R, LI Y Q, et al. Pressure-induced metallization of lead-free halide double perovskite (NH4)2PtI6 [J]. Advanced Science, 2022, 9(28): 2203442. doi: 10.1002/advs.202203442 [108] MATSUISHI K, ISHIHARA T, ONARI S, et al. Optical properties and structural phase transitions of lead-halide based inorganic-organic 3D and 2D perovskite semiconductors under high pressure [J]. Physica Status Solidi (B), 2004, 241(14): 3328–3333. doi: 10.1002/pssb.200405229 [109] YIN T T, FANG Y N, CHONG W K, et al. High-pressure-induced comminution and recrystallization of CH3NH3PbBr3 nanocrystals as large thin nanoplates [J]. Advanced Materials, 2018, 30(2): 1705017. doi: 10.1002/adma.201705017 [110] YIN T T, LIU B, YAN J X, et al. Pressure-engineered structural and optical properties of two-dimensional (C4H9NH3)2PbI4 perovskite exfoliated nm-thin flakes [J]. Journal of the American Chemical Society, 2019, 141(3): 1235–1241. doi: 10.1021/jacs.8b07765 [111] LIU S, SUN S S, GAN C K, et al. Manipulating efficient light emission in two-dimensional perovskite crystals by pressure-induced anisotropic deformation [J]. Science Advances, 2019, 5(7): eaav9445. doi: 10.1126/sciadv.aav9445 [112] GUO S H, ZHAO Y S, BU K J, et al. Pressure-suppressed carrier trapping leads to enhanced emission in two-dimensional perovskite (HA)2(GA)Pb2I7 [J]. Angewandte Chemie International Edition, 2020, 59(40): 17533–17539. doi: 10.1002/anie.202001635 [113] MA Z W, LI F F, SUI L Z, et al. Tunable color temperatures and emission enhancement in 1D halide perovskites under high pressure [J]. Advanced Optical Materials, 2020, 8(18): 2000713. doi: 10.1002/adom.202000713 [114] SHI Y, MA Z W, ZHAO D L, et al. Pressure-induced emission (PIE) of one-dimensional organic tin bromide perovskites [J]. Journal of the American Chemical Society, 2019, 141(16): 6504–6508. doi: 10.1021/jacs.9b02568 [115] REN X T, YAN X Z, AHMAD A S, et al. Pressure-induced phase transition and band gap engineering in propylammonium lead bromide perovskite [J]. The Journal of Physical Chemistry C, 2019, 123(24): 15204–15208. doi: 10.1021/acs.jpcc.9b02854 [116] LI Q, CHEN Z W, YANG B, et al. Pressure-induced remarkable enhancement of self-trapped exciton emission in one-dimensional CsCu2I3 with tetrahedral units [J]. Journal of the American Chemical Society, 2020, 142(4): 1786–1791. doi: 10.1021/jacs.9b13419 [117] LI Q, CHEN Z W, LI M Z, et al. Pressure-engineered photoluminescence tuning in zero-dimensional lead bromide trimer clusters [J]. Angewandte Chemie International Edition, 2021, 60(5): 2583–2587. doi: 10.1002/anie.202009237 [118] ZHANG L, LIU C M, LIN Y, et al. Tuning optical and electronic properties in low-toxicity organic-inorganic hybrid (CH3NH3)3Bi2I9 under high pressure [J]. The Journal of Physical Chemistry Letters, 2019, 10(8): 1676–1683. doi: 10.1021/acs.jpclett.9b00595 [119] MA Z W, LIU Z, LU S Y, et al. Pressure-induced emission of cesium lead halide perovskite nanocrystals [J]. Nature Communications, 2018, 9(1): 4506. doi: 10.1038/s41467-018-06840-8 [120] WANG T Y, DAIBER B, FROST J M, et al. Indirect to direct bandgap transition in methylammonium lead halide perovskite [J]. Energy & Environmental Science, 2017, 10(2): 509–515. doi: 10.1039/c6ee03474h [121] HAN Q F, BAE S H, SUN P Y, et al. Single crystal formamidinium lead iodide (FAPbI3): insight into the structural, optical, and electrical properties [J]. Advanced Materials, 2016, 28(11): 2253–2258. doi: 10.1002/adma.201505002 [122] MIZUSAKI J, ARAI K, FUEKI K. Ionic conduction of the perovskite-type halides [J]. Solid State Ionics, 1983, 11(3): 203–211. doi: 10.1016/0167-2738(83)90025-5 [123] YAMADA K, ISOBE K, TSUYAMA E, et al. Chloride ion conductor CH3NH3GeCl3 studied by Rietveld analysis of X-ray diffraction and 35Cl NMR [J]. Solid State Ionics, 1995, 79: 152–157. doi: 10.1016/0167-2738(95)00055-B [124] YAMADA K, KURANAGA Y, UEDA K, et al. Phase transition and electric conductivity of ASnCl3 (A= Cs and CH3NH3) [J]. Bulletin of the Chemical Society of Japan, 1998, 71(1): 127–134. doi: 10.1246/bcsj.71.127 [125] AZPIROZ J M, MOSCONI E, BISQUERT J, et al. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation [J]. Energy & Environmental Science, 2015, 8(7): 2118–2127. doi: 10.1039/c5ee01265a [126] YAN H C, OU T J, JIAO H, et al. Pressure dependence of mixed conduction and photo responsiveness in organolead tribromide perovskites [J]. The Journal of Physical Chemistry Letters, 2017, 8(13): 2944–2950. doi: 10.1021/acs.jpclett.7b01022 [127] LIN J, CHEN H, GAO Y, et al. Pressure-induced semiconductor-to-metal phase transition of a charge-ordered indium halide perovskite [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(47): 23404–23409. doi: 10.1073/pnas.1907576116 [128] YAO X D, BAI Y X, JIN C, et al. Anomalous polarization enhancement in a van der Waals ferroelectric material under pressure [J]. Nature Communications, 2023, 14(1): 4301. doi: 10.1038/s41467-023-40075-6 -








 下载:
下载:






 下载:
下载: