Laser-Induced Phase Separation of Mixed-Halide CsPb(IxBr1−x)3 Perovskite Nanocrystals under High Pressure
-
摘要: 混合卤化物类钙钛矿具有多种优异的光电特性,如随卤素成分变化而大范围可调的带隙、高荧光量子产率等,是制备太阳能电池和发光二极管等光电材料的理想候选材料。然而,混合卤化物钙钛矿的稳定性较差,如在强光照条件下会发生相分离,这种不稳定性阻碍了它们在光电领域的广泛应用,因此,研究其相分离的内在机理和控制方法对于改善其特性以实现实际应用至关重要。针对强激光照射下具有不同组分的CsPb(IxBr1−x)3纳米晶,系统研究了其激光诱导相分离随压强的变化,发现不同I/Br比例的CsPb(IxBr1−x)3纳米晶具有不同的激光诱导相分离特征:x<0.1的富溴样品随着激光照射而迅速产生CsPbBr3纯相,并实现较大的荧光量子产率增益;0.1<x<0.9溴含量较多的样品明显形成了富溴相,并产生了新的荧光峰;而x>0.9低溴含量样品则只产生荧光峰宽化,并伴随荧光强度的快速降低。将CsPb(IxBr1−x)3纳米晶置于准静水压强环境中,观察到富溴样品和较多溴含量样品中的相分离随着压强的升高而迅速减缓,并在约0.1 GPa的较低压强下被极大程度地抑制,而低溴含量样品的相分离则随压强上升而增强。这些发现为理解和克服相关光电材料在强光工作环境中的应用问题提供了一种有效的解决途径。
-
关键词:
- 压强调控 /
- 激光诱导 /
- CsPb(IxBr1–x)3相分离 /
- 原位表征
Abstract: Mixed-halide perovskites have a variety of excellent photovoltaic properties, including the band gap that is widely tunable with the halogen composition, high photoluminescence quantum yield (PLQY), and so on, making them ideal candidates for the photovoltaic device applications such as solar cells and light-emitting diodes. However, mixed-halide perovskites often encounter phase separation under light illumination, which hinders their wide application in optoelectronics. Therefore, investigating the intrinsic mechanism and controlling methods of their phase separation is crucial to improve their properties for practical applications. In this work, a systematic study of the laser-induced phase separation of CsPb(IxBr1−x)3 nanocrystals with different compositions under strong laser irradiation at different pressures was carried out. We discovered that CsPb(IxBr1−x)3 nanocrystals with different I/Br ratios possess different characteristics of laser-induced phase separation, for example, at ambient pressure, the bromine-rich samples with x<0.1 produce nearly full-bromide CsPbBr3 phase rapidly and achieve a large PLQY gain; the samples with 0.1<x<0.9 clearly form a new photoluminescence (PL) peak at lower wavelength, which represents the bromine-rich phase generation; while the samples with low bromine content with x>0.9 only produce a broadening of the PL peak as well as a rapid decrease of the PL intensity. By subjecting CsPb(IxBr1−x)3 nanocrystals to a quasi-hydrostatic pressure environment, it was observed that phase separation in bromine-rich samples (x<0.9) rapidly slowed down with increasing pressure and was largely suppressed at a mild pressure of about 0.1 GPa, while phase separation in samples with low bromine content was enhanced with increasing pressure. These findings provide an effective and practical way to understand and overcome the problem of application of relevant photoelectric devices in intense light environments. -
图 3 (a) 0.02 GPa、(b) 0.05 GPa和(c) 0.08 GPa下CsPb(I0.09Br0.91)3纳米晶的光诱导相分离,(d) 激光照射前、后PL峰位随压强的变化(黑线)以及PLQY随压强的变化(蓝线)
Figure 3. Laser-induced phase separation of CsPb(I0.09Br0.91)3 nanocrystals under (a) 0.02 GPa, (b) 0.05 GPa and (c) 0.08 GPa, respectively; (d) PL peak positions before and after laser irradiation under different pressures (black line), and the PLQY under different pressures (blue line)
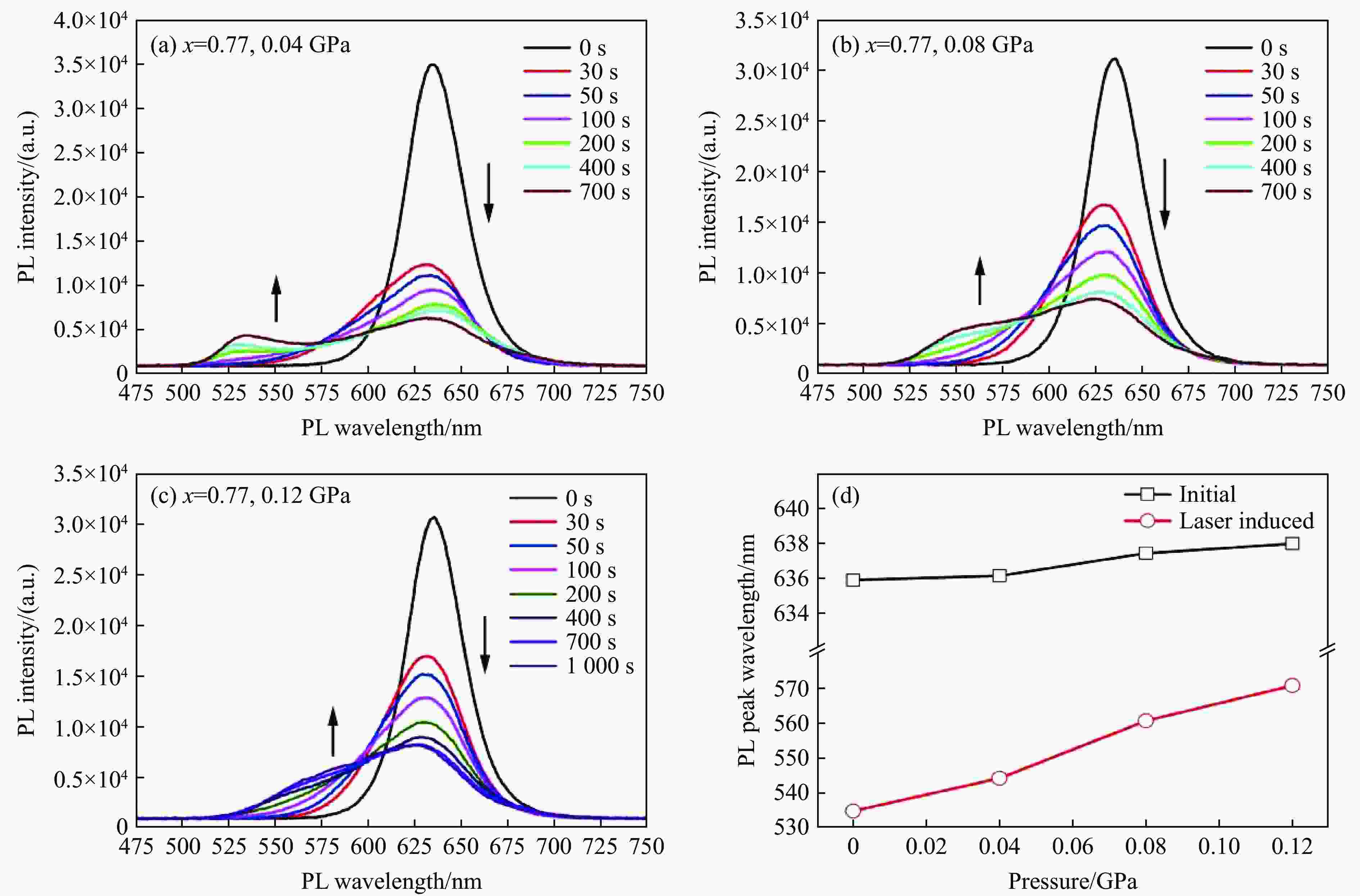
图 4 (a) 0.04 GPa、(b) 0.08 GPa和(c) 0.12 GPa下CsPb(I0.77Br0.23)3纳米晶的光诱导相分离,(d) 原PL峰位随压强的变化(黑线)和激光照射后PL峰位随压强的变化(红线)
Figure 4. Laser-induced phase separation of CsPb(I0.77Br0.23)3 nanocrystals under (a) 0.04 GPa, (b) 0.08 GPa, and (c) 0.12 GPa, respectively; (d) initial PL peak center position (black line) and new PL peak center position after laser irradiation (red line) as function of pressure
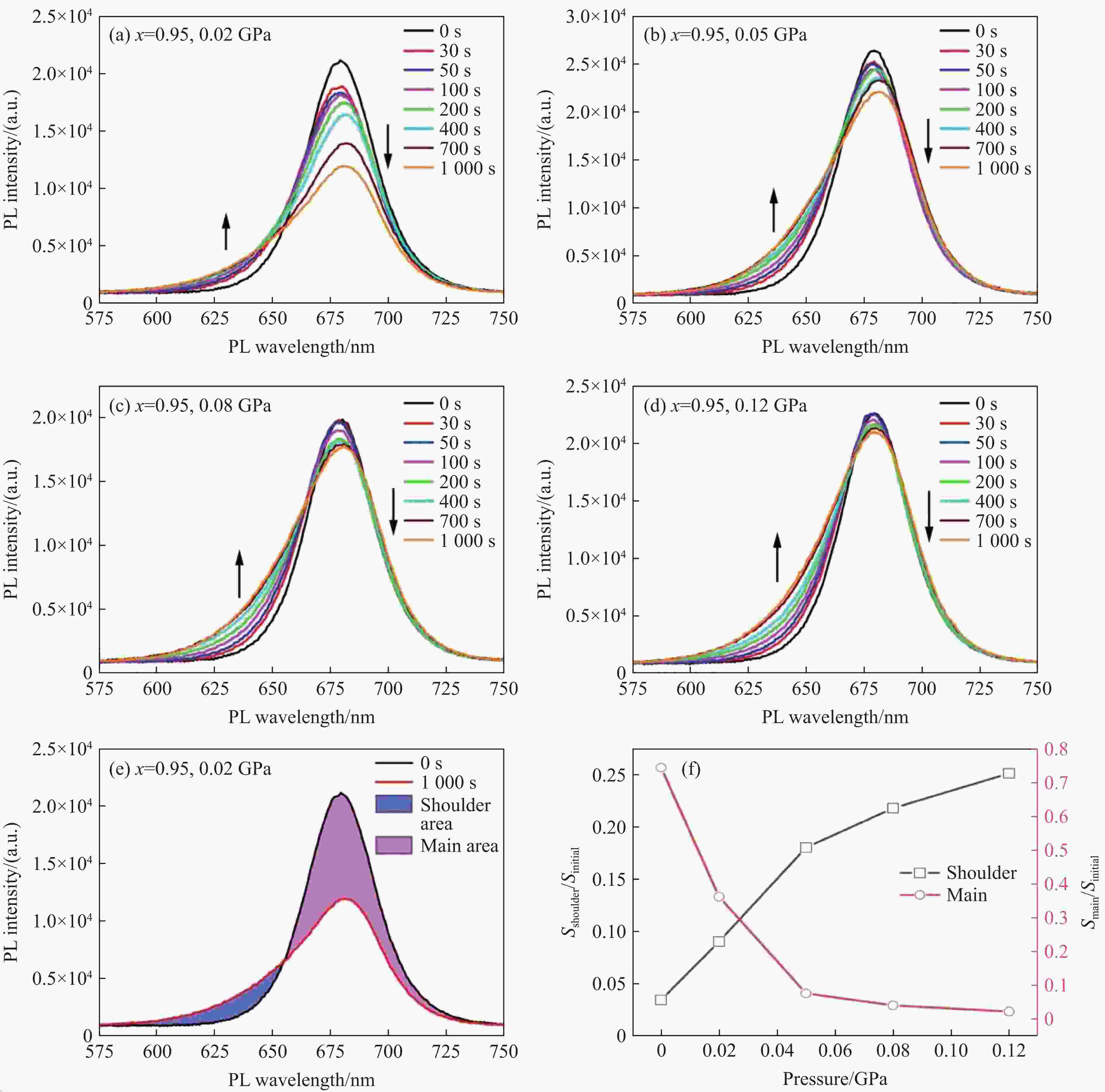
图 5 (a) 0.02 GPa、(b) 0.05 GPa、(c) 0.08 GPa和(d) 0.12 GPa下CsPb(I0.95Br0.05)3纳米晶的光诱导相分离,(e) 0.02 GPa下CsPb(I0.95Br0.05)3纳米晶在0 s(黑线)与
1000 s(红线)时的PL数据对比(蓝色区域为PL峰在激光照射后向低波长处的宽化拖尾,粉色区域为激光照射后主PL峰的强度下降),(f) 激光照射1000 s后PL峰在低波长处的宽化拖尾面积(黑线)以及强度下降面积(红线)与照射前PL峰面积的比值随压强的变化Figure 5. Laser-induced phase separation of CsPb(I0.95Br0.05)3 nanocrystals under (a) 0.02 GPa, (b) 0.05 GPa, (c) 0.08 GPa and (d) 0.12 GPa, respectively; (e) comparison of PL data of CsPb(I0.95Br0.05)3 nanocrystals at 0 s (black line) versus
1000 s (red line) under 0.02 GPa (The blue area represents the PL peak broadened shoulder toward the lower wavelength after laser irradiation, and the pink area represents the intensity drop of the main PL peak after laser irradiation); (f) trend of the ratio of the broadened shoulder area (black line) and the decreased area of the main PL peak (red line) after laser irradiation for1000 s to the initial PL peaks before irradiation with pressure -
[1] NOH J H, IM S H, HEO J H, et al. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells [J]. Nano Letters, 2013, 13(4): 1764–1769. doi: 10.1021/nl400349b [2] WANG Z, SHI Z J, LI T T, et al. Stability of perovskite solar cells: a prospective on the substitution of the A cation and X anion [J]. Angewandte Chemie International Edition, 2017, 56(5): 1190–1212. doi: 10.1002/anie.201603694 [3] AHMAD S, KANAUJIA P K, BEESON H J, et al. Strong photocurrent from two-dimensional excitons in solution-processed stacked perovskite semiconductor sheets [J]. ACS Applied Materials & Interfaces, 2015, 7(45): 25227–25236. doi: 10.1021/acsami.5b07026 [4] HOKE E T, SLOTCAVAGE D J, DOHNER E R, et al. Reversible photo-induced trap formation in mixed-halide hybrid perovskites for photovoltaics [J]. Chemical Science, 2015, 6(1): 613–617. doi: 10.1039/C4SC03141E [5] REHMAN W, MILOT R L, EPERON G E, et al. Charge-carrier dynamics and mobilities in formamidinium lead mixed-halide perovskites [J]. Advanced Materials, 2015, 27(48): 7938–7944. doi: 10.1002/adma.201502969 [6] BEAL R E, SLOTCAVAGE D J, LEIJTENS T, et al. Cesium lead halide perovskites with improved stability for tandem solar cells [J]. The Journal of Physical Chemistry Letters, 2016, 7(5): 746–751. doi: 10.1021/acs.jpclett.6b00002 [7] BUSH K A, FROHNA K, PRASANNA R, et al. Compositional engineering for efficient wide band gap perovskites with improved stability to photoinduced phase segregation [J]. ACS Energy Letters, 2018, 3(2): 428–435. doi: 10.1021/acsenergylett.7b01255 [8] ZHANG H C, FU X, TANG Y, et al. Phase segregation due to ion migration in all-inorganic mixed-halide perovskite nanocrystals [J]. Nature Communications, 2019, 10(1): 1088. doi: 10.1038/s41467-019-09047-7 [9] FUNK H, SHARGAIEVA O, ELJARRAT A, et al. In situ TEM monitoring of phase-segregation in inorganic mixed halide perovskite [J]. The Journal of Physical Chemistry Letters, 2020, 11(13): 4945–4950. doi: 10.1021/acs.jpclett.0c01296 [10] WANG Y G, LÜ X J, YANG W G, et al. Pressure-induced phase transformation, reversible amorphization, and anomalous visible light response in organolead bromide perovskite [J]. Journal of the American Chemical Society, 2015, 137(34): 11144–11149. doi: 10.1021/jacs.5b06346 [11] LI M, LIU T B, WANG Y G, et al. Pressure responses of halide perovskites with various compositions, dimensionalities, and morphologies [J]. Matter and Radiation at Extremes, 2020, 5(1): 018201. doi: 10.1063/1.5133653 [12] CHEN M T, GUO S H, BU K J, et al. Pressure-induced robust emission in a zero-dimensional hybrid metal halide (C9NH20)6Pb3Br12 [J]. Matter and Radiation at Extremes, 2021, 6(5): 058401. doi: 10.1063/5.0058821 [13] MUSCARELLA L A, HUTTER E M, WITTMANN F, et al. Lattice compression increases the activation barrier for phase segregation in mixed-halide perovskites [J]. ACS Energy Letters, 2020, 5(10): 3152–3158. doi: 10.1021/acsenergylett.0c01474 [14] JAFFE A, LIN Y, BEAVERS C M, et al. High-pressure single-crystal structures of 3D lead-halide hybrid perovskites and pressure effects on their electronic and optical properties [J]. ACS Central Science, 2016, 2(4): 201–209. doi: 10.1021/acscentsci.6b00055 [15] PROTESESCU L, YAKUNIN S, BODNARCHUK M I, et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut [J]. Nano Letters, 2015, 15(6): 3692–3696. doi: 10.1021/nl5048779 [16] TOBY B H. EXPGUI, a graphical user interface for GSAS [J]. Journal of Applied Crystallography, 2001, 34(2): 210–213. doi: 10.1107/S0021889801002242 [17] BERTOLOTTI F, PROTESESCU L, KOVALENKO M V, et al. Coherent nanotwins and dynamic disorder in cesium lead halide perovskite nanocrystals [J]. ACS Nano, 2017, 11(4): 3819–3831. doi: 10.1021/acsnano.7b00017 [18] MAO H K, XU J, BELL P M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions [J]. Journal of Geophysical Research: Solid Earth, 1986, 91(B5): 4673–4676. doi: 10.1029/JB091iB05p04673 [19] DIASPRO A, CHIRICO G, USAI C, et al. Photobleaching [M]//PAWLEY J B. Handbook of Biological Confocal Microscopy. 3rd ed. New York: Springer, 2006: 690–702. [20] ZHANG L, ZENG Q X, WANG K. Pressure-induced structural and optical properties of inorganic halide perovskite CsPbBr3 [J]. The Journal of Physical Chemistry Letters, 2017, 8(16): 3752–3758. doi: 10.1021/acs.jpclett.7b01577 [21] BEIMBORN J C II, HALL L M G, TONGYING P, et al. Pressure response of photoluminescence in cesium lead iodide perovskite nanocrystals [J]. The Journal of Physical Chemistry C, 2018, 122(20): 11024–11030. doi: 10.1021/acs.jpcc.8b03280 [22] WANG Z W, ZENG L W, ZHU T, et al. Suppressed phase segregation for triple-junction perovskite solar cells [J]. Nature, 2023, 618(7963): 74–79. doi: 10.1038/s41586-023-06006-7 [23] ZHAO Y C, MIAO P, ELIA J, et al. Strain-activated light-induced halide segregation in mixed-halide perovskite solids [J]. Nature Communications, 2020, 11(1): 6328. doi: 10.1038/s41467-020-20066-7 -







 下载:
下载: