Recent Progress on Structural and Functional Evolutions of Metal Halide Perovskites under High Pressure
-
摘要: 过去10年里,金属卤素钙钛矿作为一种性能优异的新型功能材料被广泛应用,其研究取得了很多重要进展。压力作为一个基本的热力学变量,可以显著地影响材料的微观结构、原子间相互作用、电子轨道和化学键,是调节材料结构和性能的一个强大工具。与此同时,压力也为研究结构与性质之间的关系提供了新路径。结合金刚石对顶砧高压装置以及原位高压表征技术,总结了金属卤素钙钛矿在高压下的结构及性质变化,包括高压驱动结构相变,有序-无序转变,非晶化,局部结构演化,带隙、光致发光、光响应、电阻等性质在压力作用下的变化,以及高压下特有的奇特性质如金属化转变,系统分析了此类材料的结构-性质关系,并对未来的新型材料设计做出了展望。Abstract: Over the past decade, metal halide perovskites have been widely employed as the emerging active-materials for technological innovations, and their research has become one of the central goals in the field of energetic materials. Pressure, a new thermodynamic dimension, can tune microstructure, atomic interactions, electronic orbitals, and chemical bonds of materials, thus serves as a potent means to regulate the structures and properties of metal halide perovskites. In addition, pressure paves a novel avenue for probing and understanding the structure-property relationship. Taking the advantage of diamond anvil cell technology and in situ high-pressure characterization techniques, we have comprehensively summarized the pressure-induced evolutions of metal halide perovskites, encompassing structural phase transitions, order-disorder transitions, amorphization, and local structural evolution. We have examined alterations in properties, such as bandgap, photoluminescence, photoelectronic response, and electrical resistance, and other distinctive high-pressure phenomena. This review systematically analyzes the structure-property interplay within these known materials, and offers insights into the design of future novel materials.
-
Key words:
- high-pressure /
- metal halide perovskite /
- structural evolution /
- semiconductor /
- diamond anvil cell
-
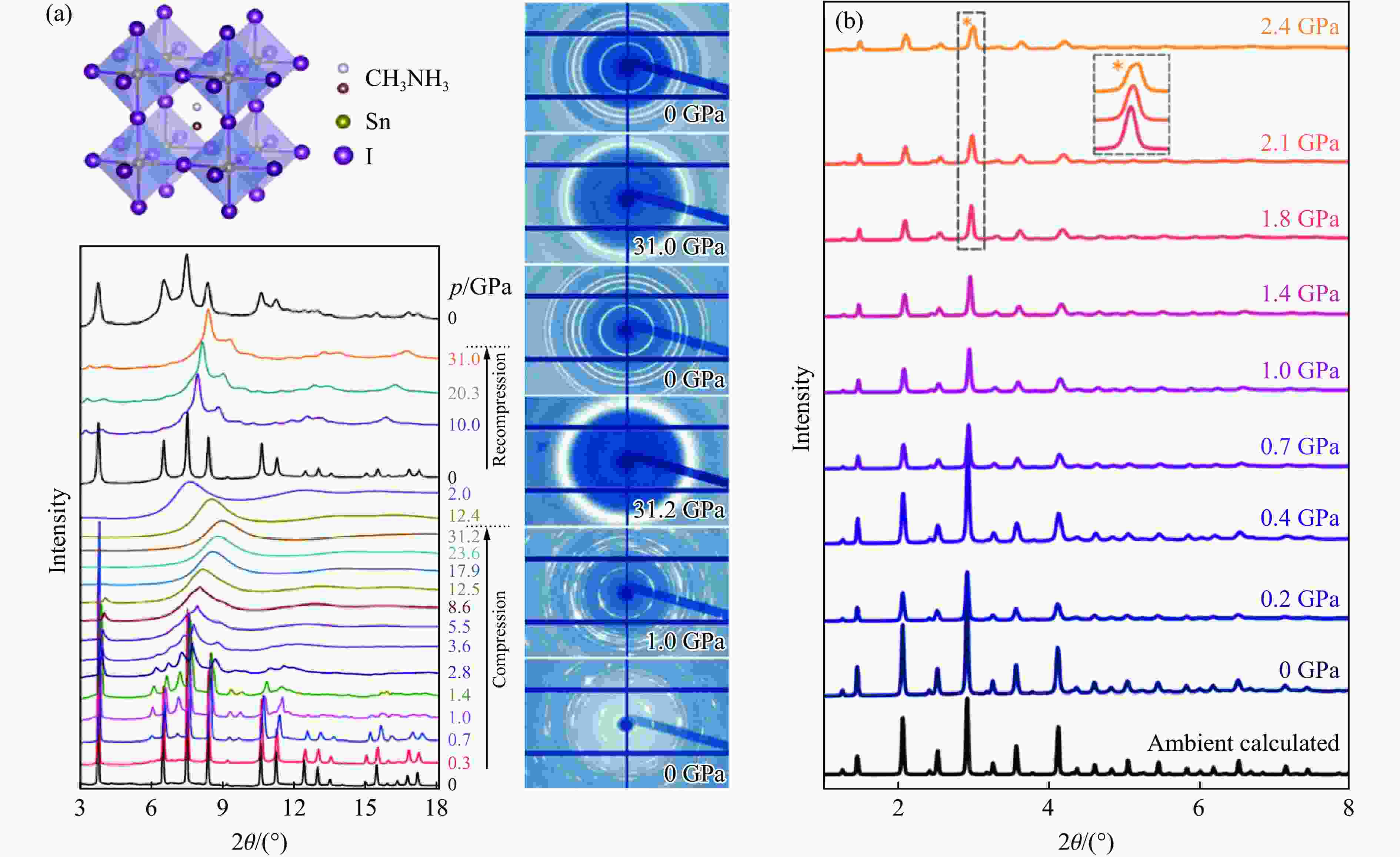
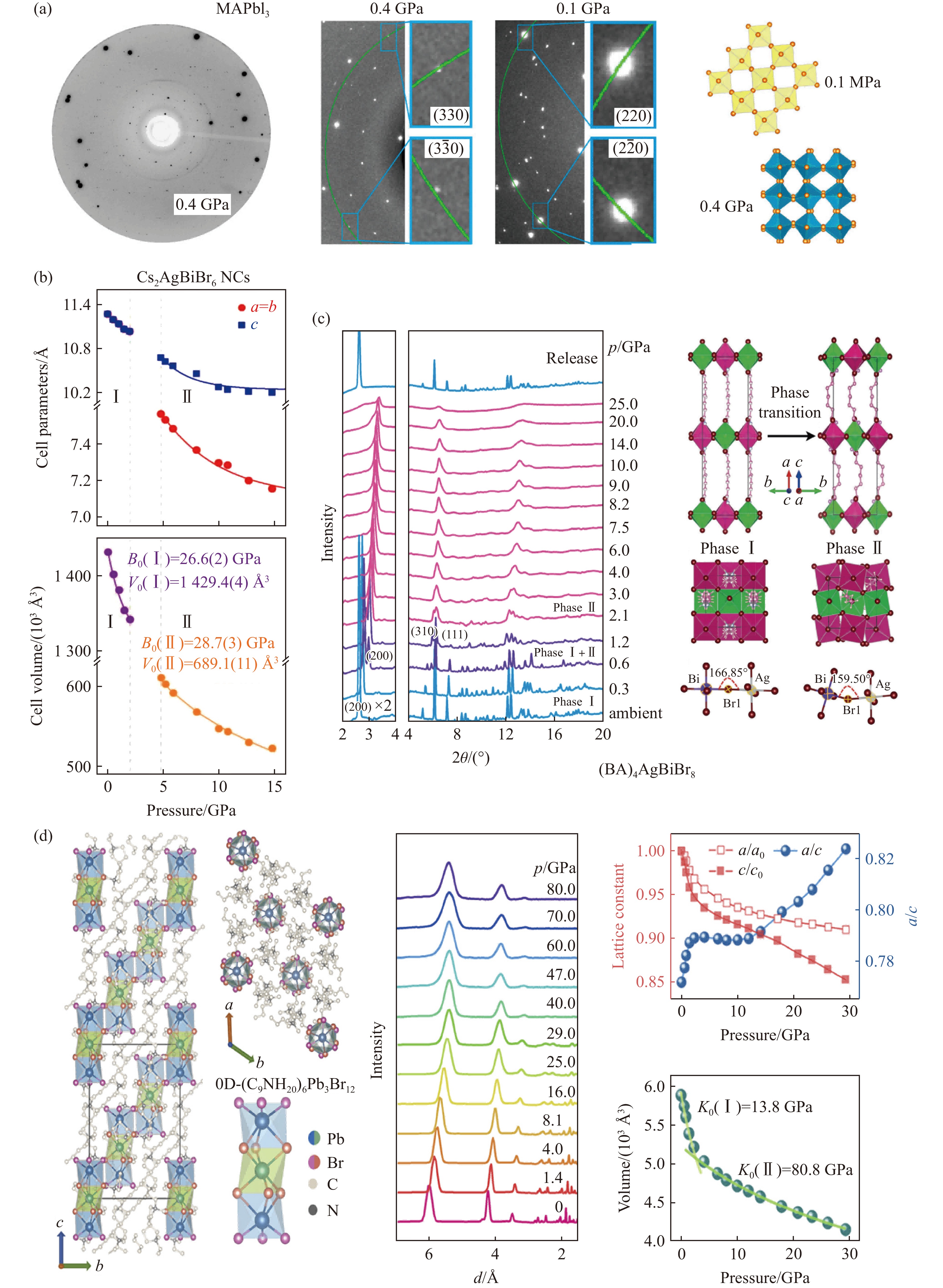
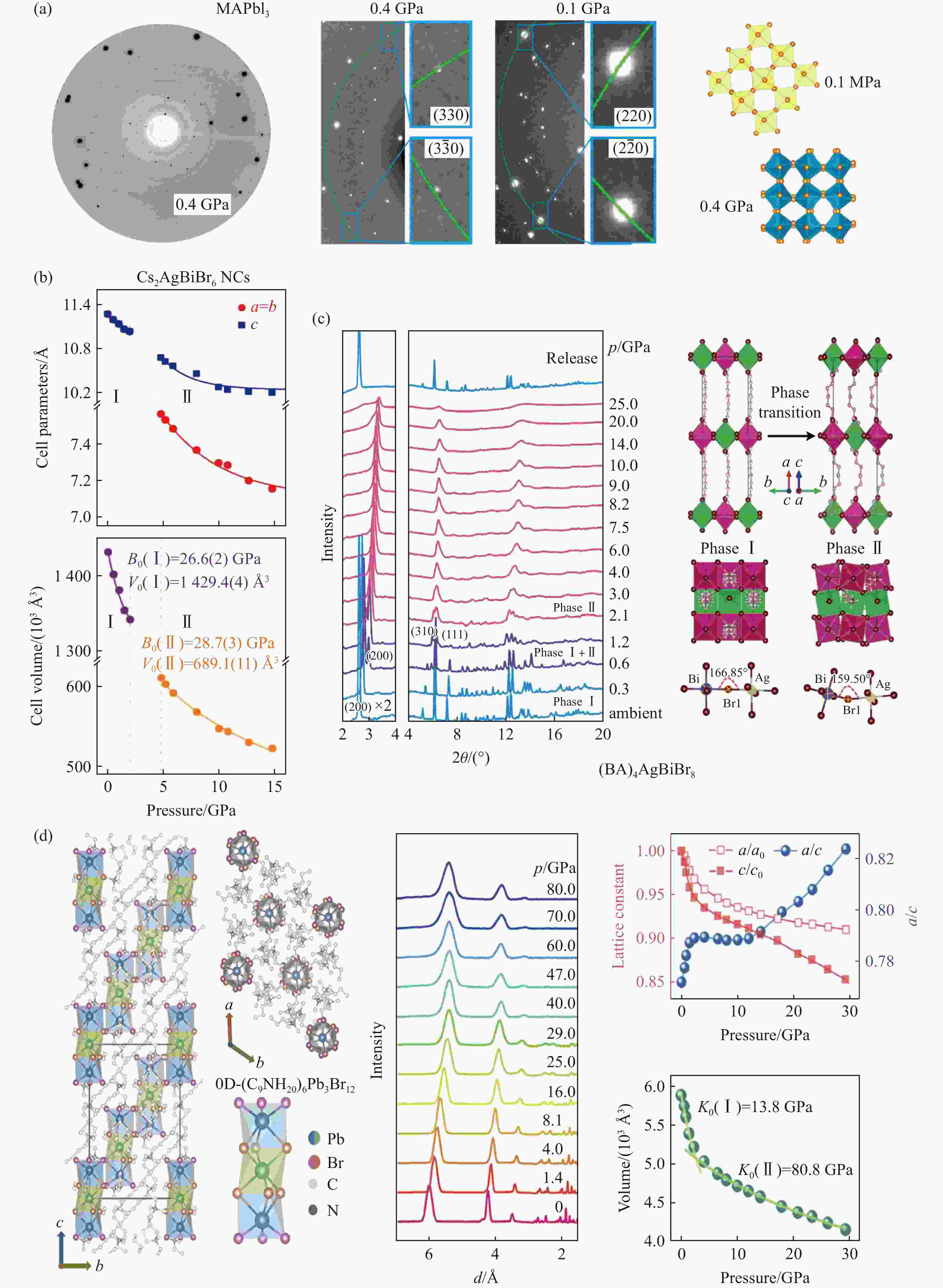
图 2 MHPVs中压力驱动的非晶化、有序-无序转变、局部无序:(a) MASnI3在压缩和再压缩时压力驱动的结构演化[25],(b) Cs2AgBiBr6在2.1 GPa时隐藏的局部无序[36]
Figure 2. Pressure-driven amorphous, ordered-disordered transition, local disorder: (a) pressure-driven structural evolution of MASnI3 during compression and recompression in MHPVs[25];(b) Cs2AgBiBr6 local disorder at 2.1 GPa[36]
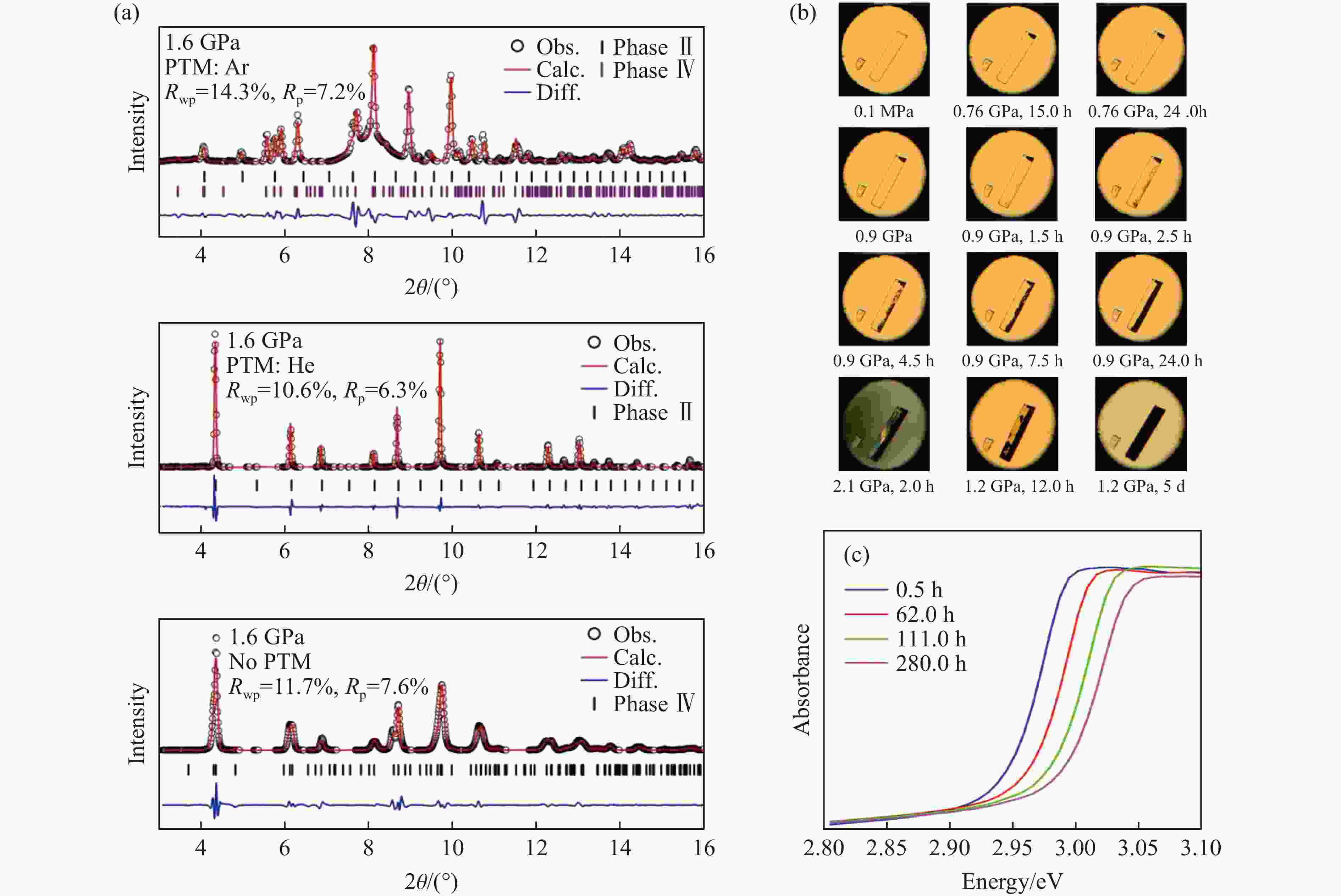
图 3 高压实验条件及时间对结构和性能的影响:(a)不同传压介质(pressure-transmitting-medium,PTM)下MAPbBr3在同一压力时的结构[15];高压下MAPbCl3的结构(b)和性能(c)的时间依赖[14]
Figure 3. Effect of high-pressure experimental conditions and time on structure and performance: (a) the structure of MAPbBr3 at the same pressure under different pressure-transmitting-media[15]; time dependence of structure (b) and performance (c) of MAPbCl3 under high pressure[14]
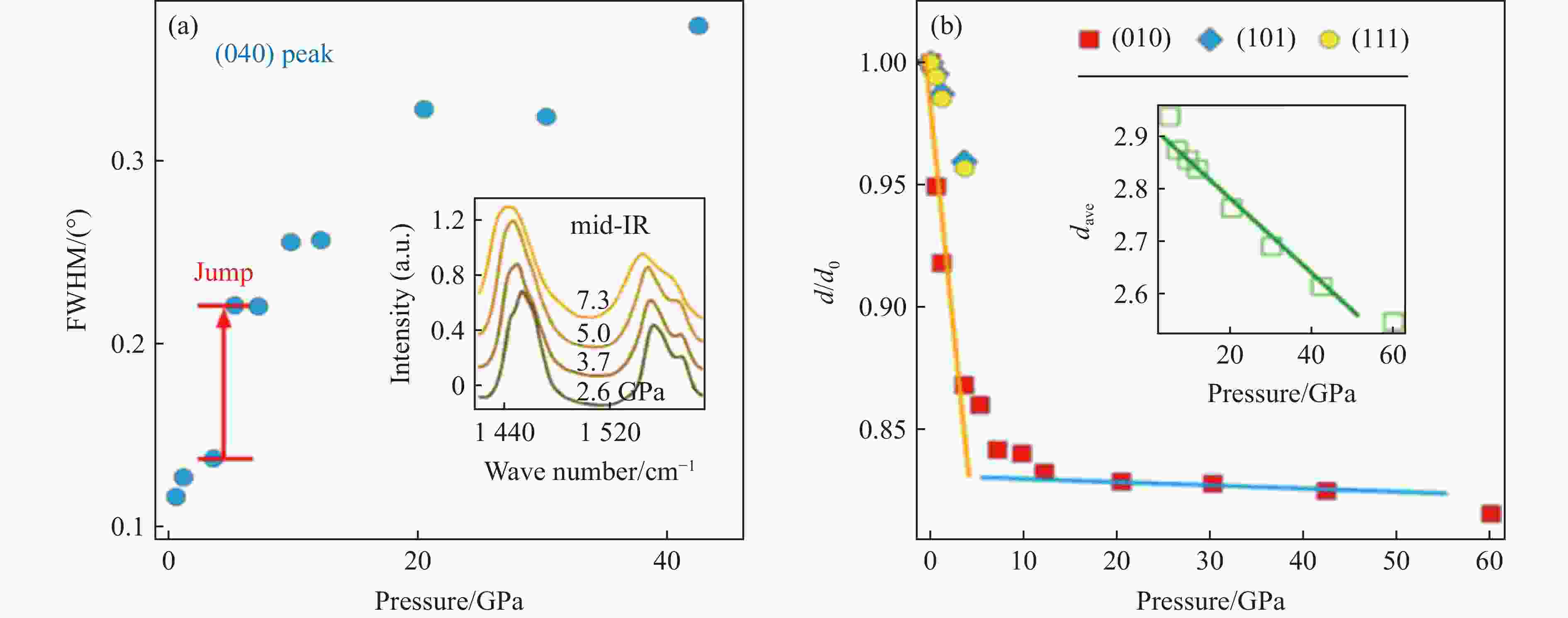
图 4 (a)~(b) 结合中红外峰证明压力下的有序-无序转变[69],其中,FWHM为半峰宽,d为晶面间距,d0为常压下的晶面间距
Figure 4. (a)−(b) order-disordered transitions under pressure demonstrated by FWHM and d/d0 and mid-infrared peaks,where FWHM represent full width at half maximum, d represents the interplanar spacing under the current pressure, and d0 represents the interplanar spacing under ambient[69]
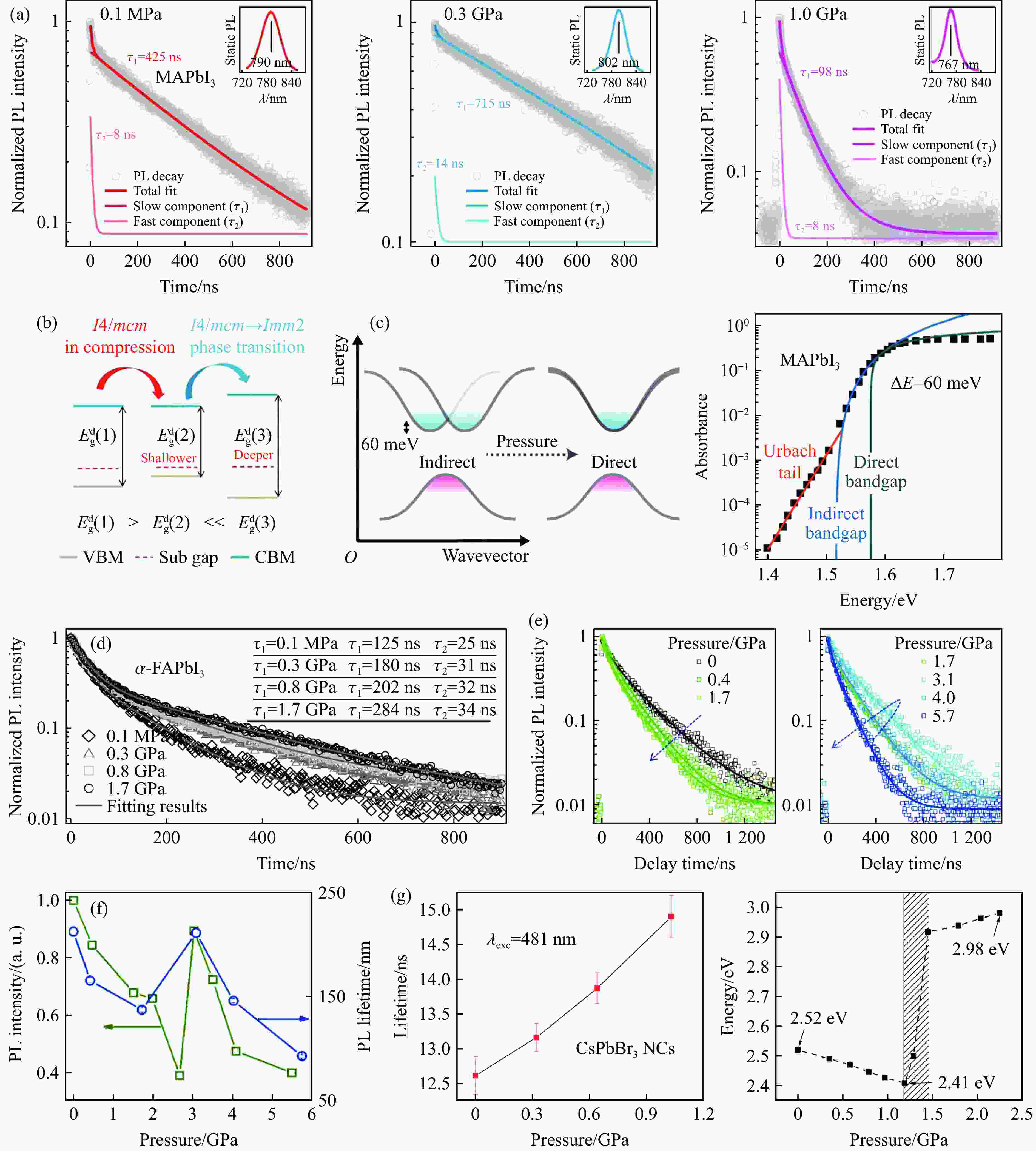
图 5 带隙的压力依赖性:(a) 不同卤素钙钛矿中的带隙演化[11, 26, 31, 41, 42, 55, 63, 69, 76, 85, 89, 97, 105, 107],(b) 压缩下Pb―I―Pb键的键长和键角的变化[68]
Figure 5. Pressure dependence of band gap:(a) band gap evolution in different halogen perovskites[11, 26, 31, 41, 42, 55, 63, 69, 76, 85, 89, 97, 105, 107]; (b) changes of bond length and bond angle of Pb―I―Pb bonds under compression[68]
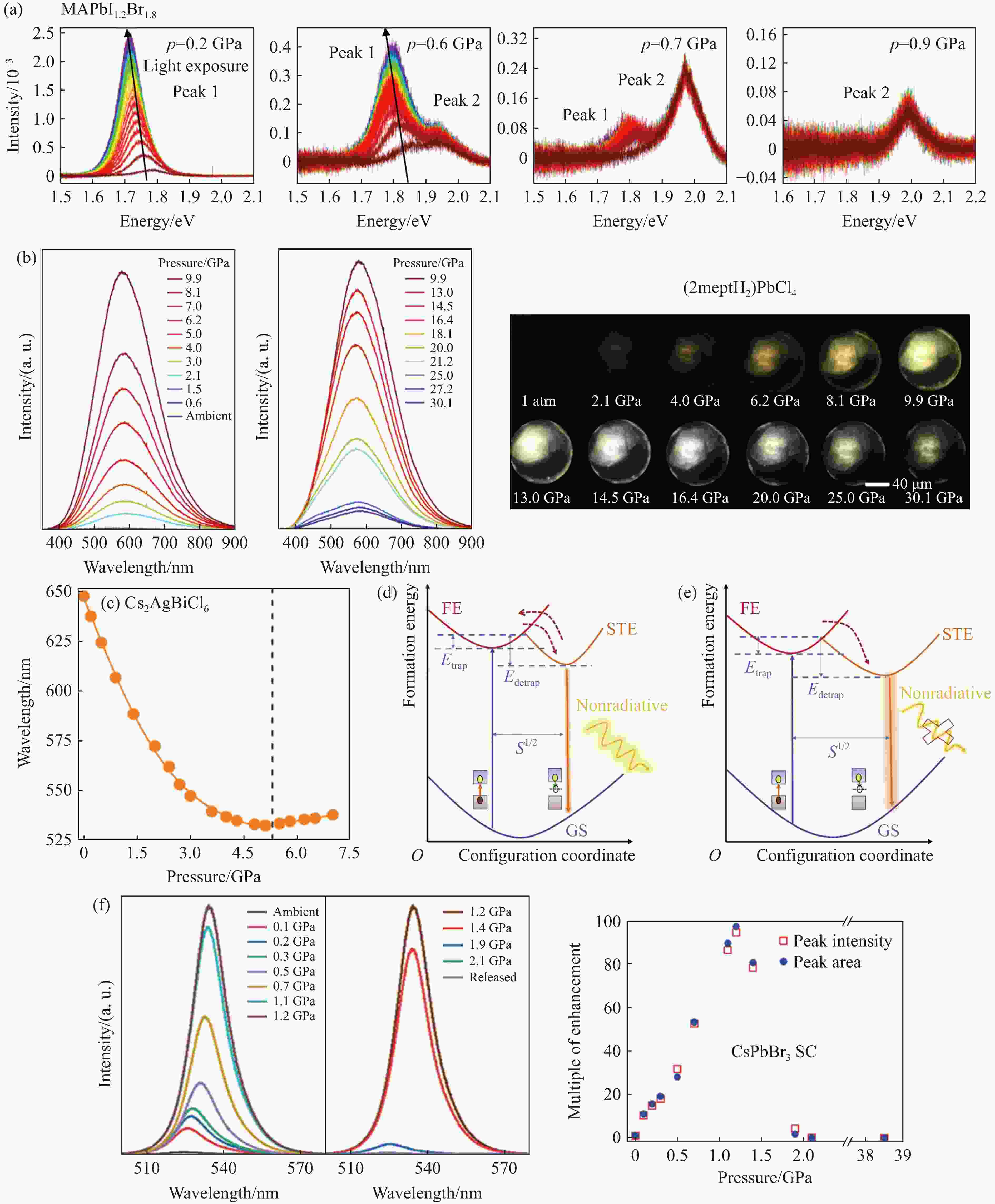
图 6 不同MHPVs PL的压力依赖:(a) MAPbI1.2Br1.8[9]的PL谱,(b) (2meptH2)PbCl4钙钛矿的PL压力依赖和压力下的光学图像[43],(c) Cs2AgBiCl6[96]的PL谱,(d)~(e)常温常压和高压下自捕获激子发射的演化示意图[96],(f) CsPbBr3单晶的PL压力依赖[106]
Figure 6. Pressure dependence of different MHPVs PL: (a) MAPbI1.2Br1.8[9]; (b) pressure dependence of PL and optical image of (2meptH2)PbCl4[43]; (c) Cs2AgBiCl6[96]; (d)–(e) schematic diagram of the evolution of self-captured exciton emission under environmental conditions and high pressure[38];(f) pressure dependence of PL in CsPbBr3 single crystal[106];
图 7 (a) MAPbI3的压力依赖PL衰减动力学[11],(b) MAPbI3靠近价带顶的缺陷态在压力下变浅[11],(c) MAPbI3的弱间接带隙[120],(d) α-FAPbI3的压力依赖PL衰减动力学[26],(e) 不同压力下MAPbI3 MC的压力依赖PL衰减动力学[19],(f) MAPbI3 MC平均PL寿命和PL强度的压力依赖[19],(g) CsPbBr3 NCs载流子寿命和带隙的压力依赖[29]
Figure 7. (a) MAPbI3 pressure-dependent PL attenuation kinetics[11]; (b) pressure dependence of defect states in MAPbI3[11]; (c) weak indirect bandgap of MAPbI3[120]; (d) α-FAPbI3 pressure-dependent PL attenuation kinetics[26]; (e) pressure-dependent PL decay kinetics of MAPbI3 MC[19]; (f) pressure dependence of average PL lifetime and PL intensity of MAPbI3 MC[19]; (g) pressure dependence of carrier lifetime and bandgap in CsPbBr3 NCs[29]
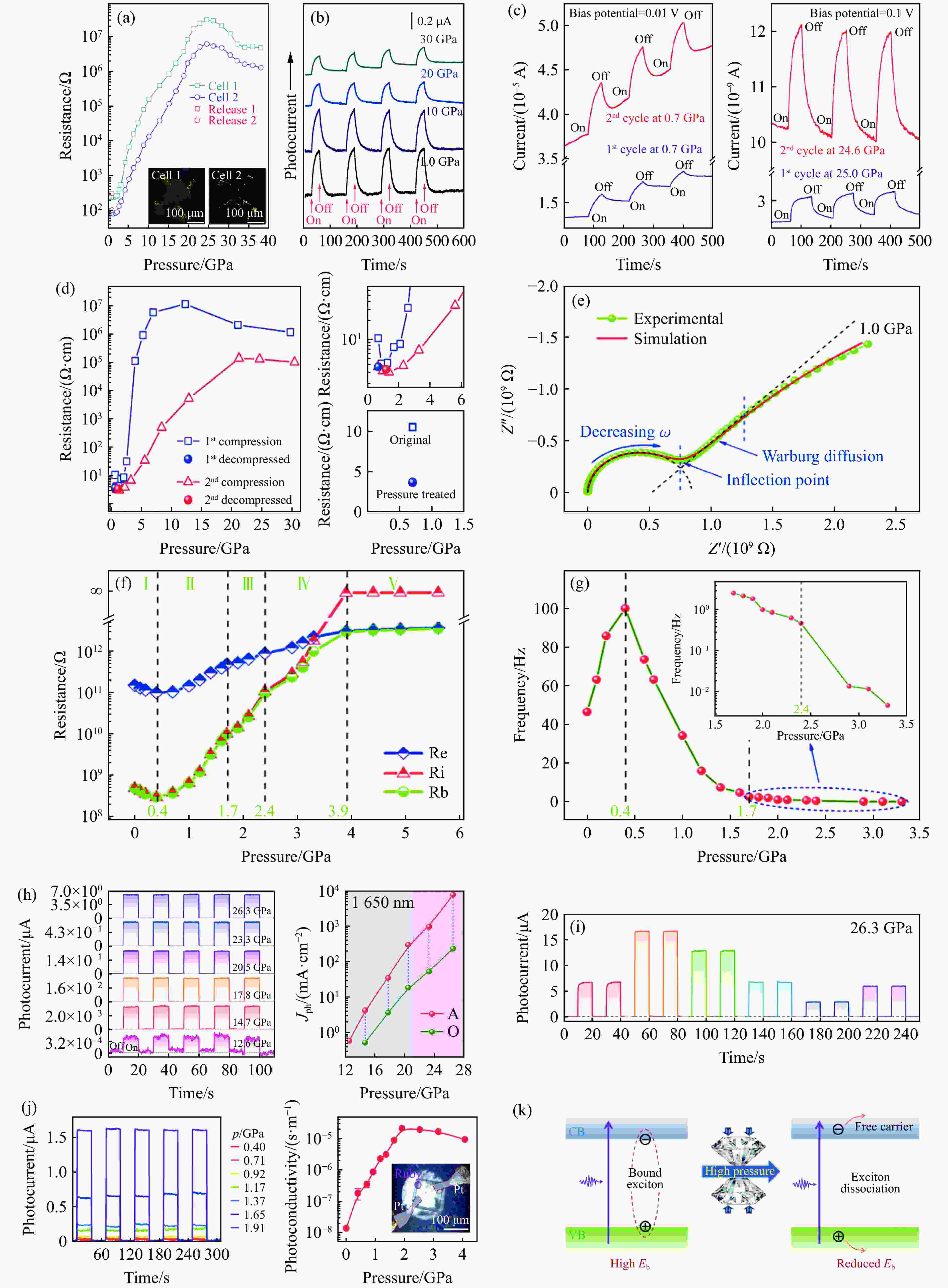
图 8 压力驱动的不同MHPVs的光电性质的演化:(a)~(b) MAPbBr3[23],(c)~(d) MASnI3[25],(e)~(g) MAPbBr3多晶的电输运性能[126],(h)~(i) Cs3Bi2I9的增强的光电流和宽带光响应[65],(j) Cs2PbI2Cl2在2 GPa下的显著的光电流增强[44],(k)压力促进的激子解离示意图[44]
Figure 8. Pressure-driven evolution of photoelectric properties of different MHPVs: (a)–(b) MAPbBr3[23]; (c)–(d)MASnI3[25];(e)–(g) electrical transport performance of MAPbBr3 polycrystalline [126]; (h)–(i) enhanced photocurrent and broadband light response of Cs3Bi2I9[65]; (j) significant photocurrent enhancement at 2 GPa for Cs2PbI2Cl2[44]; (k) schematic diagram of stress-facilitated exciton dissociation[44]
图 9 高压下MHPVs的金属化:(a)~(b) MAPbI3带隙的压力依赖[13],(c) 高压下MAPbI3的红外反射光谱[13],(d) 高压下MAPbI3的电导率的温度依赖[13],(e) Cs2In(Ⅰ)In(Ⅲ)Cl6高压原位拉曼光谱[127],(f) Cs2In(I)In(III)Cl6的压力依赖光吸收光谱[127],(g)~(h) 压力下CD3ND3PbI3的红外吸收光谱[18],(i) 72 GPa压力下CD3ND3PbI3的带隙为零[18]
Figure 9. MHPVs metallization under high pressure: (a)–(b) pressure dependence of the MAPbI3 bandgap[13]; (c) infrared reflectance spectrum of MAPbI3 at high pressure[13]; (d) temperature dependence of MAPbI3 conductivity at high pressure[13]; (e) high-pressure in situ Raman of Cs2In(Ⅰ)In(Ⅲ )Cl6[127]; (f) pressure-dependent light absorption spectra of Cs2In(Ⅰ)In(Ⅲ )Cl6[127];(g)–(h) CD3ND3PbI3 IR absorption spectra under pressure[18]; (i) CD3ND3PbI3 with zero bandgap at 72 GPa[18]
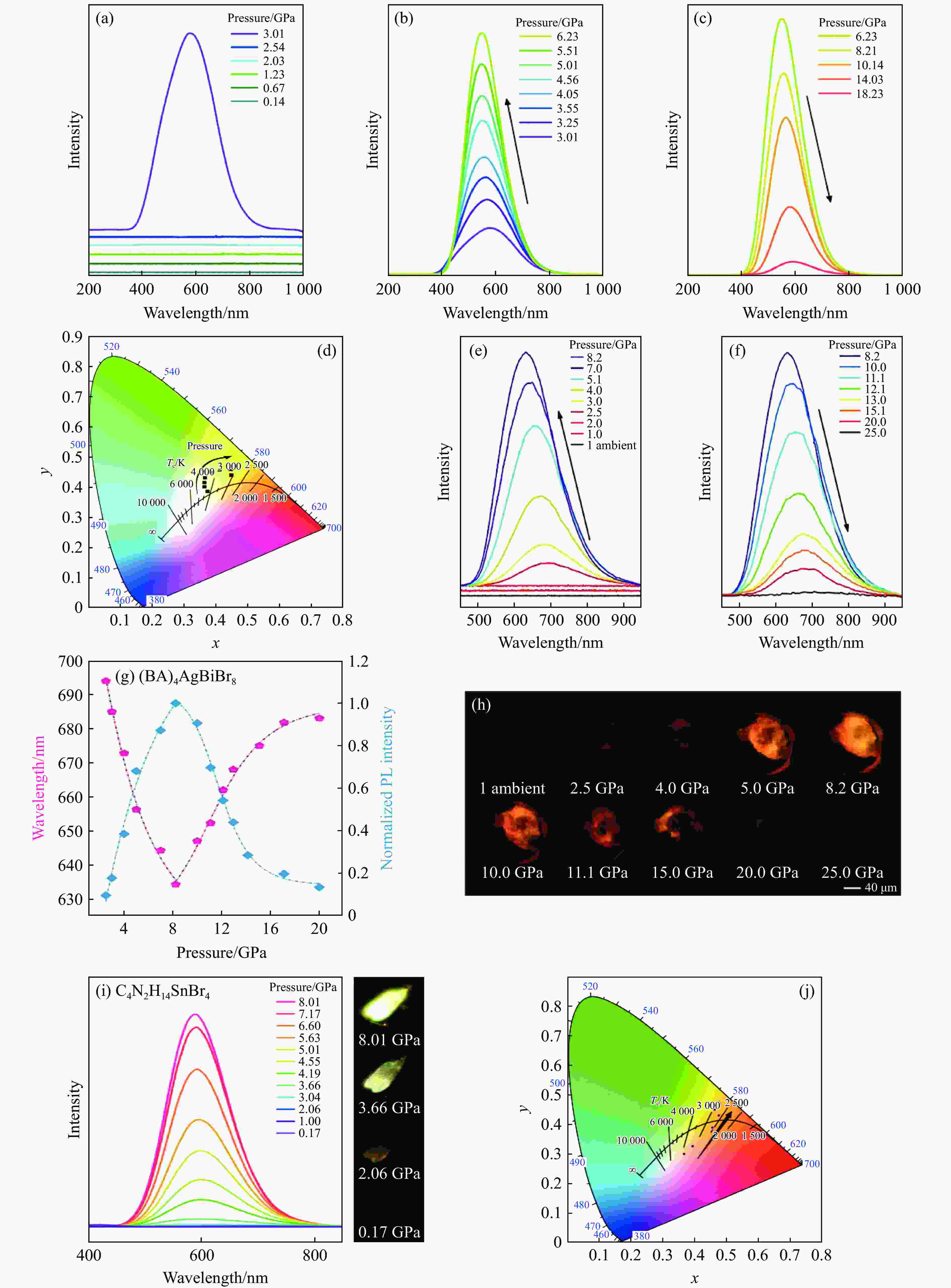
图 10 不同MHPVs的压致发光行为:(a)~(c) Cs4PbBr6 NCs从3.01 GPa开始表现出明显的光发射[119],(d) Cs4PbBr6 NCs的与压力相关的色度坐标[119],(e)~(f) (BA)4AgBiBr8在高压下的PL光谱[39],(g) (BA)4AgBiBr8的PL位置和PL强度的压力依赖[39],(h) (BA)4AgBiBr8在高压下的光学图像(PL随压力的增加而变化)[39],(i)~(j) C4N2H14SnBr4的压力依赖PL光谱和与压力相关的色度坐标[114]
Figure 10. Pressed luminescence of different MHPVS:(a)–(c) Cs4PbBr6 nanocrystals begin to exhibit significant emission at high pressure of 3.01GPa[119]; (d) pressure-dependent chromaticity coordinates of Cs4PbBr6 nanocrystals[119] ; (e)–(f) PL spectroscopy of (BA)4AgBiBr8 at high pressure[39]; (g) pressure dependence of PL position and PL strength of (BA)4AgBiBr8[39]; (h) the optical pattern of (BA)4AgBiBr8 at high pressure shows that PL varies with increasing pressure[39]; (i)–(j) pressure dependent PL spectrum and pressure-dependent chromaticity coordinates of C4N2H14SnBr4[114]
表 1 金属卤化物钙钛矿的压力诱导相变
Table 1. Pressure-induced phase transitions of metal halide perovskite
Material Phase transitions Ref. MAPbBr3 Pm$ \overline 3 $m (ambient pressure)→Im$ \overline 3 $ (0.4 GPa)→Pnma (1.8 GPa) [23] MAPbI3 I4/mcm (ambient pressure)→Imm2 (0.26 GPa) [8] MAPbCl3 Pm$ \overline 3 $m (ambient pressure)→Pm$ \overline 3 $m (0.8 GPa)→Pnma (2.0 GPa) [12] CD3ND3PbI3 I4/mcm (ambient pressure)→Imm2 (1.30 GPa)→Imm (2.57 GPa) [18] MASnI3 P4mm (ambient pressure)→Pnma (0.7 GPa) [25] MAPbI1.2Br1.8 Pm$ \overline 3 $m (ambient pressure)→Im$ \overline 3 $ (2.7 GPa) [9] MASnCl3 Pc (ambient pressure)→P1 (1 GPa)→amorphization (above 3 GPa) [24] MA3Bi2Br9 P$ \overline 3 $m1 (ambient pressure)→P21/a (5 GPa) [64] FAPbBr3 Pm$ \overline 3 $m (ambient pressure)→Im$ \overline 3 $ (0.53 GPa)→Pnma (2.2 GPa) [90] FAPbI3 No phase transitions below 7 GPa [26] FAPbI3 NCs Pm$ \overline 3 $m (ambient pressure)→Im$ \overline 3 $(0.6 GPa) [27] α-FAPbI3 Pm$ \overline 3 $m→Imm2 (0.3 GPa), Imm2→Immm (1.7 GPa) [28] (C9NH20)6Pb3Br12 No phase transitions below 80 GPa [38] DABCuCl4 P21/a (ambient pressure)→P2 (6.4 GPa) [63] BA2PbI4 Pbca (ambient pressure)→P21/a (2 GPa) [22] MHy2PbBr4 Pmn21→P21 (near 4 GPa) [74] Cy4BiBr7 No phase transitions below 20.13 GPa [84] CsPbBr3 Isostructural phase transition (about 1.2 GPa) [29] CsPbBr3 Isostructural phase transition (1.2 GPa) [98] CsPbBr3 Pbnm (ambient pressure)→Pm3m (1.7 GPa) [33] RP-CsPbBr3 Pbnm (ambient pressure)→P21/m (0.74 GPa ) [33] CsPbI3 Pnma (ambient pressure)→P21/m (5.6 GPa) [30] Cs2SnBr6 No phase transitions below 20 GPa [34] Cs2AgBiBr6 Fm$ \overline 3 $m (ambient pressure)→I4/m (4.5 GPa) [99] Cs3Bi2I9 No phase transitions below 12.7 GPa [97] Cs3Bi2I9 No phase transitions below 20.3 GPa [93] Cs3Bi2Br9 P$ \overline 3 $m1 (ambient pressure)→C2/c (10.1 GPa) [37] Cs2AgBiCl6 Fm$ \overline 3 $m (ambient pressure)→I4/m (5.6 GPa) [96] Cs2PbI2Cl2 I4/mmm (ambient pressure)→C2/m (2.8 GPa) [44] 表 2 不同MHPVs的PL发生和消失压力
Table 2. Pressures corresponding PL occurrence and disappearance for different MHPVs
Material Dimension Initial pressure of PL/GPa PL annihilation pressure/GPa Ref. MAPbCl3 3D Ambient 7.20 [108] MAPbBr3 3D Ambient 4.85 [108] MAPbBr3 3D Ambient 4.00 [109] MAPbI3 3D Ambient 2.70 [10] MAPbI1.2Br1.8 3D Ambient 1.60 [9] CsPb2Br5 3D Ambient 2.23 [40] CsPbBr3 3D Ambient 2.40 [106] Cs2AgBiCl6 3D Ambient 8.00 [96] (BA)2PbI4 2D Ambient 10.00 [110] (BA)2PbI4 2D Ambient 12.60 [22] (PEA)2PbBr4 2D Ambient 15.60 [41] (PEA)2PbI4 2D Ambient 7.60 [111] (HA)2(GA)Pb2I7 2D Ambient 9.48 [112] (BA)2(MA)Pb2I7 2D Ambient 4.70 [69] (GA)(MA)2Pb2I7 2D Ambient 7.00 [46] (BA)4AgBiBr8 2D 2.50 25.00 [39] C4N2H14PbBr4 1D Ambient 9.00 [91] C4N2H14PbBr4 1D Ambient 24.81 [113] C4N2H14SnBr4 1D 2.06 20.02 [114] CH3(CH2)2NH3PbBr3 1D Ambient 7.30 [115] CsCu2I3 1D Ambient 16.00 [116] (bmpy)9[ZnBr4]2[Pb3Br11] 0D Ambient 18.20 [117] (bmpy)6[Pb3Br12] 0D Ambient >80 [38] (MA)3Bi2I9 0D Ambient 9.00 [118] Cs4PbBr6 0D 3.01 18.23 [119] Cs3Bi2I9 0D Ambient 9.30 [97] -
[1] TRAVIS W, GLOVER E N K, BRONSTEIN H, et al. On the application of the tolerance factor to inorganic and hybrid halide perovskites: a revised system [J]. Chemical Science, 2016, 7(7): 4548–4556. doi: 10.1039/C5SC04845A [2] KIESLICH G, SUN S J, CHEETHAM A K. An extended tolerance factor approach for organic-inorganic perovskites [J]. Chemical Science, 2015, 6(6): 3430–3433. doi: 10.1039/C5SC00961H [3] BOYD C C, CHEACHAROEN R, LEIJTENS T, et al. Understanding degradation mechanisms and improving stability of perovskite photovoltaics [J]. Chemical Reviews, 2019, 119(5): 3418–3451. doi: 10.1021/acs.chemrev.8b00336 [4] Best research-cell efficiency chart [EB/OL]. [2023-10-15]. https://www.nrel.gov/pv/cell-efficiency.html. [5] CHEN X Y, XIE J J, WANG W, et al. Research progress of compositional controlling strategy to perovskite for high performance solar cells [J]. Acta Chimica Sinica, 2019, 77(1): 9–23. doi: 10.6023/a18100447 [6] LIU G, KONG L P, YANG W G, et al. Pressure engineering of photovoltaic perovskites [J]. Materials Today, 2019, 27: 91–106. doi: 10.1016/j.mattod.2019.02.016 [7] KOJIMA A, TESHIMA K, SHIRAI Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells [J]. Journal of the American Chemical Society, 2009, 131(17): 6050–6051. doi: 10.1021/ja809598r [8] CAPITANI F, MARINI C, CARAMAZZA S, et al. High-pressure behavior of methylammonium lead iodide (MAPbI3) hybrid perovskite [J]. Journal of Applied Physics, 2016, 119(18): 185901. doi: 10.1063/1.4948577 [9] JAFFE A, LIN Y, BEAVERS C M, et al. High-pressure single-crystal structures of 3D lead-halide hybrid perovskites and pressure effects on their electronic and optical properties [J]. ACS Central Science, 2016, 2(4): 201–209. doi: 10.1021/acscentsci.6b00055 [10] JIANG S J, FANG Y N, LI R P, et al. Pressure-dependent polymorphism and band-gap tuning of methylammonium lead iodide perovskite [J]. Angewandte Chemie International Edition, 2016, 55(22): 6540–6544. doi: 10.1002/anie.201601788 [11] KONG L P, LIU G, GONG J, et al. Simultaneous band-gap narrowing and carrier-lifetime prolongation of organic-inorganic trihalide perovskites [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(32): 8910–8915. doi: 10.1073/pnas.1609030113 [12] WANG L R, WANG K, XIAO G J, et al. Pressure-induced structural evolution and band gap shifts of organometal halide perovskite-based methylammonium lead chloride [J]. The Journal of Physical Chemistry Letters, 2016, 7(24): 5273–5279. doi: 10.1021/acs.jpclett.6b02420 [13] JAFFE A, LIN Y, MAO W L, et al. Pressure-induced metallization of the halide perovskite (CH3NH3)PbI3 [J]. Journal of the American Chemical Society, 2017, 139(12): 4330–4333. doi: 10.1021/jacs.7b01162 [14] SZAFRAŃSKI M, KATRUSIAK A. Photovoltaic hybrid perovskites under pressure [J]. The Journal of Physical Chemistry Letters, 2017, 8(11): 2496–2506. doi: 10.1021/acs.jpclett.7b00520 [15] ZHANG R, CAI W Z, BI T G, et al. Effects of nonhydrostatic stress on structural and optoelectronic properties of methylammonium lead bromide perovskite [J]. The Journal of Physical Chemistry Letters, 2017, 8(15): 3457–3465. doi: 10.1021/acs.jpclett.7b01367 [16] JIANG H Y, XUE H T, WANG L F, et al. Effect of pressure-induced structural phase transition on electronic and optical properties of perovskite CH3NH3PbI3 [J]. Materials Science in Semiconductor Processing, 2019, 96: 59–65. doi: 10.1016/j.mssp.2019.01.038 [17] LEE J H, JAFFE A, LIN Y, et al. Origins of the pressure-induced phase transition and metallization in the halide perovskite (CH3NH3)PbI3 [J]. ACS Energy Letters, 2020, 5(7): 2174–2181. doi: 10.1021/acsenergylett.0c00772 [18] KONG L P, GONG J, HU Q Y, et al. Suppressed lattice disorder for large emission enhancement and structural robustness in hybrid lead iodide perovskite discovered by high-pressure isotope effect [J]. Advanced Functional Materials, 2021, 31(9): 2009131. doi: 10.1002/adfm.202009131 [19] YIN Y F, TIAN W M, LUO H, et al. Excellent carrier transport property of hybrid perovskites sustained under high pressures [J]. ACS Energy Letters, 2022, 7(1): 154–161. doi: 10.1021/acsenergylett.1c02359 [20] JUNG Y K, ABDULLA M, FRIEND R H, et al. Pressure-induced non-radiative losses in halide perovskite light-emitting diodes [J]. Journal of Materials Chemistry C, 2022, 10(35): 12560–12568. doi: 10.1039/D2TC01490D [21] OU T J, YAN J J, XIAO C H, et al. Visible light response, electrical transport, and amorphization in compressed organolead iodine perovskites [J]. Nanoscale, 2016, 8(22): 11426–11431. doi: 10.1039/C5NR07842C [22] YUAN Y, LIU X F, MA X D, et al. Large band gap narrowing and prolonged carrier lifetime of (C4H9NH3)2PbI4 under High Pressure [J]. Advanced Science, 2019, 6(15): 1900240. doi: 10.1002/advs.201900240 [23] WANG Y G, LYU X J, YANG W G, et al. Pressure-induced phase transformation, reversible amorphization, and anomalous visible light response in organolead bromide perovskite [J]. Journal of the American Chemical Society, 2015, 137(34): 11144–11149. doi: 10.1021/jacs.5b06346 [24] WANG L R, OU T J, WANG K, et al. Pressure-induced structural evolution, optical and electronic transitions of nontoxic organometal halide perovskite-based methylammonium tin chloride [J]. Applied Physics Letters, 2017, 111(23): 233901. doi: 10.1063/1.5004186 [25] LÜ X J, WANG Y G, STOUMPOS C C, et al. Enhanced structural stability and photo responsiveness of CH3NH3SnI3 perovskite via pressure-induced amorphization and recrystallization [J]. Advanced Materials, 2016, 28(39): 8663–8668. doi: 10.1002/adma.201600771 [26] LIU G, KONG L P, GONG J, et al. Pressure-induced bandgap optimization in lead-based perovskites with prolonged carrier lifetime and ambient retainability [J]. Advanced Functional Materials, 2017, 27(3): 1604208. doi: 10.1002/adfm.201604208 [27] ZHU H, CAI T, QUE M D, et al. Pressure-induced phase transformation and band-gap engineering of formamidinium lead iodide perovskite nanocrystals [J]. The Journal of Physical Chemistry Letters, 2018, 9(15): 4199–4205. doi: 10.1021/acs.jpclett.8b01852 [28] WANG P, GUAN J W, GALESCHUK D T K, et al. Pressure-induced polymorphic, optical, and electronic transitions of formamidinium lead iodide perovskite [J]. The Journal of Physical Chemistry Letters, 2017, 8(10): 2119–2125. doi: 10.1021/acs.jpclett.7b00665 [29] XIAO G J, CAO Y, QI G Y, et al. Pressure effects on structure and optical properties in cesium lead bromide perovskite nanocrystals [J]. Journal of the American Chemical Society, 2017, 139(29): 10087–10094. doi: 10.1021/jacs.7b05260 [30] YUAN G, QIN S, WU X, et al. Pressure-induced phase transformation of CsPbI3 by X-ray diffraction and Raman spectroscopy [J]. Phase Transitions, 2018, 91(1): 38–47. doi: 10.1080/01411594.2017.1357180 [31] CAO Y, QI G Y, LIU C, et al. Pressure-tailored band gap engineering and structure evolution of cubic cesium lead iodide perovskite nanocrystals [J]. The Journal of Physical Chemistry C, 2018, 122(17): 9332–9338. doi: 10.1021/acs.jpcc.8b01673 [32] LIANG Y F, HUANG X L, HUANG Y P, et al. New metallic ordered phase of perovskite CsPbI3 under pressure [J]. Advanced Science, 2019, 6(14): 1900399. doi: 10.1002/advs.201900399 [33] YESUDHAS S, MORRELL M V, ANDERSON M J, et al. Pressure-induced phase changes in cesium lead bromide perovskite nanocrystals with and without Ruddlesden-Popper faults [J]. Chemistry of Materials, 2020, 32(2): 785–794. doi: 10.1021/acs.chemmater.9b04157 [34] YUAN G, HUANG S X, NIU J J, et al. Compressibility of Cs2SnBr6 by X-ray diffraction and Raman spectroscopy [J]. Solid State Communications, 2018, 275: 68–72. doi: 10.1016/j.ssc.2018.03.014 [35] FU R J, CHEN Y P, YONG X, et al. Pressure-induced structural transition and band gap evolution of double perovskite Cs2AgBiBr6 nanocrystals [J]. Nanoscale, 2019, 11(36): 17004–17009. doi: 10.1039/C9NR07030C [36] GIRDZIS S P, LIN Y, LEPPERT L, et al. Revealing local disorder in a silver-bismuth halide perovskite upon compression [J]. The Journal of Physical Chemistry Letters, 2021, 12(1): 532–536. doi: 10.1021/acs.jpclett.0c03412 [37] GENG T, WEI S, ZHAO W Y, et al. Insight into the structure-property relationship of two-dimensional lead-free halide perovskite Cs3Bi2Br9 nanocrystals under pressure [J]. Inorganic Chemistry Frontiers, 2021, 8(6): 1410–1415. doi: 10.1039/D0QI01300E [38] FANG Y Y, ZHANG L, WU L W, et al. Pressure-induced emission (PIE) and phase transition of a two-dimensional halide double perovskite (BA)4AgBiBr8 (BA=CH3(CH2)3NH3+) [J]. Angewandte Chemie International Edition, 2019, 58(43): 15249–15253. doi: 10.1063/5.0058821 [39] CHEN M T, GUO S H, BU K J, et al. Pressure-induced robust emission in a zero-dimensional hybrid metal halide (C9NH20)6Pb3Br12 [J]. Matter and Radiation at Extremes, 2021, 6(5): 08401. doi: 10.1002/anie.201906311 [40] MA Z W, LI F F, QI G Y, et al. Structural stability and optical properties of two-dimensional perovskite-like CsPb2Br5 microplates in response to pressure [J]. Nanoscale, 2019, 11(3): 820–825. doi: 10.1039/C8NR05684F [41] ZHANG L, WU L W, WANG K, et al. Pressure-induced broadband emission of 2D organic-inorganic hybrid perovskite (C6H5C2H4NH3)2PbBr4 [J]. Advanced Science, 2019, 6(2): 1801628. doi: 10.1002/advs.201801628 [42] ZHAN X H, JIANG X M, LV P, et al. Enhanced structural stability and pressure-induced photoconductivity in two-dimensional hybrid perovskite (C6H5CH2NH3)2 CuBr4 [J]. Angewandte Chemie International Edition, 2022, 61(28): e202205491. doi: 10.1002/anie.202205491 [43] FANG Y Y, WANG J T, ZHANG L, et al. Tailoring the high-brightness “warm” white light emission of two-dimensional perovskite crystals via a pressure-inhibited nonradiative transition [J]. Chemical Science, 2023, 14(10): 2652–2658. doi: 10.1039/D2SC06982B [44] GUO S H, BU K J, LI J W, et al. Enhanced photocurrent of all-inorganic two-dimensional perovskite Cs2PbI2Cl2 via pressure-regulated excitonic features [J]. Journal of the American Chemical Society, 2021, 143(6): 2545–2551. doi: 10.1021/jacs.0c11730 [45] AZEEM M, QIN Y, LI Z G, et al. Cooperative B-site octahedral tilting, distortion and A-site conformational change induced phase transitions of a 2D lead halide perovskite [J]. Materials Chemistry Frontiers, 2021, 5(20): 7587–7594. doi: 10.1039/D1QM00566A [46] CHEN Y P, FU R J, WANG L R, et al. Emission enhancement and bandgap retention of a two-dimensional mixed cation lead halide perovskite under high pressure [J]. Journal of Materials Chemistry A, 2019, 7(11): 6357–6362. doi: 10.1039/C8TA11992A [47] CODURI M, STROBEL T A, SZAFRANSKI M, et al. Band gap engineering in MASnBr3 and CsSnBr3 perovskites: mechanistic insights through the application of pressure [J]. The Journal of Physical Chemistry Letters, 2019, 10(23): 7398–7405. doi: 10.1021/acs.jpclett.9b03046 [48] COHEN B E, WIERZBOWSKA M, ETGAR L. High efficiency and high open circuit voltage in quasi 2D perovskite based solar cells [J]. Advanced Functional Materials, 2017, 27(5): 1604733. doi: 10.1002/adfm.201604733 [49] FU R J, CHEN Y P, WANG L R, et al. Stability and band gap engineering of silica-confined lead halide perovskite nanocrystals under high pressure [J]. Geoscience Frontiers, 2021, 12(2): 957–963. doi: 10.1016/j.gsf.2020.07.004 [50] FU R J, ZHAO W Y, WANG L R, et al. Pressure-induced emission toward harvesting cold white light from warm white light [J]. Angewandte Chemie International Edition, 2021, 60(18): 10082–10088. doi: 10.1002/anie.202015395 [51] GAO F F, LI X, QIN Y, et al. Dual-stimuli-responsive photoluminescence of enantiomeric two-dimensional lead halide perovskites [J]. Advanced Optical Materials, 2021, 9(23): 2100003. doi: 10.1002/adom.202100003 [52] GAO F F, SONG H P, LI Z G, et al. Pressure-tuned multicolor emission of 2D lead halide perovskites with ultrahigh color purity [J]. Angewandte Chemie International Edition, 2023, 62(12): e202218675. doi: 10.1002/anie.202218675 [53] GAO X J, WANG Q, ZHANG Y, et al. Pressure effects on optoelectronic properties of CsPbBr3 nanocrystals [J]. The Journal of Physical Chemistry C, 2020, 124(20): 11239–11247. doi: 10.1021/acs.jpcc.0c02701 [54] GENG T, MA Z W, CHEN Y P, et al. Bandgap engineering in two-dimensional halide perovskite Cs3Sb2I9 nanocrystals under pressure [J]. Nanoscale, 2020, 12(3): 1425–1431. doi: 10.1039/C9NR09533K [55] GENG T, SHI Y, LIU Z, et al. Pressure-induced emission from all-inorganic two-dimensional vacancy-ordered lead-free metal halide perovskite nanocrystals [J]. The Journal of Physical Chemistry Letters, 2022, 13(50): 11837–11843. doi: 10.1021/acs.jpclett.2c03332 [56] GHOSH D, AZIZ A, DAWSON J A, et al. Putting the squeeze on lead iodide perovskites: pressure-induced effects to tune their structural and optoelectronic behavior [J]. Chemistry of Materials, 2019, 31(11): 4063–4071. doi: 10.1021/acs.chemmater.9b00648 [57] GHOSH P S, PONOMAREVA I. Negative linear compressibility in organic-inorganic hybrid perovskite [NH2NH3]X(HCOO)3 (X = Mn, Fe, Co) [J]. The Journal of Physical Chemistry Letters, 2022, 13(13): 3143–3149. doi: 10.1021/acs.jpclett.2c00288 [58] HUANG J S, YUAN Y B, SHAO Y C, et al. Understanding the physical properties of hybrid perovskites for photovoltaic applications [J]. Nature Reviews Materials, 2017, 2(7): 17042. doi: 10.1038/natrevmats.2017.42 [59] KE F, WANG C X, JIA C J, et al. Preserving a robust CsPbI3 perovskite phase via pressure-directed octahedral tilt [J]. Nature Communications, 2021, 12(1): 461. doi: 10.1038/s41467-020-20745-5 [60] KHOLIL M I, BHUIYAN M T H. Effects of pressure on narrowing the band gap, visible light absorption, and semi-metallic transition of lead-free perovskite CsSnBr3 for optoelectronic applications [J]. Journal of Physics and Chemistry of Solids, 2021, 154: 110083. doi: 10.1016/j.jpcs.2021.110083 [61] LI H, QIN Y, SHAN B H, et al. Unusual pressure-driven phase transformation and band renormalization in 2D vdW hybrid lead halide perovskites [J]. Advanced Materials, 2020, 32(12): 1907364. doi: 10.1002/adma.201907364 [62] LI H, WINES D, CHEN B, et al. Abnormal phase transition and band renormalization of guanidinium-based organic-inorganic hybrid perovskite [J]. ACS Applied Materials & Interfaces, 2021, 13(37): 44964–44971. doi: 10.1021/acsami.1c14521 [63] LI Q, LI S R, WANG K, et al. High-pressure study of perovskite-like organometal halide: band-gap narrowing and structural evolution of [NH3-(CH2)4-NH3]CuCl4 [J]. The Journal of Physical Chemistry Letters, 2017, 8(2): 500–506. doi: 10.1021/acs.jpclett.6b02786 [64] LI Q, YIN L X, CHEN Z W, et al. High pressure structural and optical properties of two-dimensional hybrid halide perovskite (CH3NH3)3Bi2Br9 [J]. Inorganic Chemistry, 2019, 58(2): 1621–1626. doi: 10.1021/acs.inorgchem.8b03190 [65] LI Z L, JIA B X, FANG S X, et al. Pressure-tuning photothermal synergy to optimize the photoelectronic properties in amorphous halide perovskite Cs3Bi2I9 [J]. Advanced Science, 2023, 10(6): 2205837. doi: 10.1002/advs.202205837 [66] LIANG Y F, WU M, TIAN C, et al. Pressure-tuned quantum well configuration in two-dimensional PA8Pb5I18 perovskites for highly efficient yellow fluorescence [J]. ACS Applied Energy Materials, 2021, 4(9): 10003–10011. doi: 10.1021/acsaem.1c01925 [67] LIANG Y F, ZANG Y F, HUANG X L, et al. Broadband emission enhancement induced by self-trapped excited states in one-dimensional EAPbI3 perovskite under pressure [J]. The Journal of Physical Chemistry C, 2020, 124(16): 8984–8991. doi: 10.1021/acs.jpcc.0c01553 [68] LIU G, GONG J, KONG L P, et al. Isothermal pressure-derived metastable states in 2D hybrid perovskites showing enduring bandgap narrowing [J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(32): 8076–8081. doi: 10.1073/pnas.1809167115 [69] LIU G, KONG L P, GUO P J, et al. Two regimes of bandgap red shift and partial ambient retention in pressure-treated two-dimensional perovskites [J]. ACS Energy Letters, 2017, 2(11): 2518–2524. doi: 10.1021/acsenergylett.7b00807 [70] LLOYD A J, HESTER B R, BAXTER S J, et al. Hybrid double perovskite containing helium: [He2][CaZr]F6 [J]. Chemistry of Materials, 2021, 33(9): 3132–3138. doi: 10.1021/acs.chemmater.0c04782 [71] LÜ X J, YANG W G, JIA Q X, et al. Pressure-induced dramatic changes in organic-inorganic halide perovskites [J]. Chemical Science, 2017, 8(10): 6764–6776. doi: 10.1039/C7SC01845B [72] MA Y L, ZHANG L, TANG Y, et al. Pressure-induced piezochromism and structure transitions in lead-free layered Cs4MnBi2Cl12 quadruple perovskite [J]. ACS Applied Energy Materials, 2021, 4(8): 7513–7518. doi: 10.1021/acsaem.1c01583 [73] MA Z W, LI Q, LUO J J, et al. Pressure-driven reverse intersystem crossing: new path toward bright deep-blue emission of lead-free halide double perovskites [J]. Journal of the American Chemical Society, 2021, 143(37): 15176–15184. doi: 10.1021/jacs.1c06207 [74] MĄCZKA M, SOBCZAK S, RATAJCZYK P, et al. Pressure-driven phase transition in two-dimensional perovskite MHy2PbBr4 [J]. Chemistry of Materials, 2022, 34(17): 7867–7877. doi: 10.1021/acs.chemmater.2c01533 [75] NICHOLAS A D, ZHAO J, SLEBODNICK C, et al. High-pressure structural and optical property evolution of a hybrid indium halide perovskite [J]. Journal of Solid State Chemistry, 2021, 300: 122262. doi: 10.1016/j.jssc.2021.122262 [76] RATTÉ J, MACINTOSH M F, DILORETO L, et al. Spacer-dependent and pressure-tuned structures and optoelectronic properties of 2D hybrid halide perovskites [J]. The Journal of Physical Chemistry Letters, 2023, 14(2): 403–412. doi: 10.1021/acs.jpclett.2c03555 [77] SAEED M, ALI M A, MURAD S, et al. Pressure induced structural, electronic, optical and thermal properties of CsYbBr3, a theoretical investigation [J]. Journal of Materials Research and Technology, 2021, 10: 687–696. doi: 10.1016/j.jmrt.2020.12.052 [78] SAMANTA D, SAHA P, GHOSH B, et al. Pressure-induced emergence of visible luminescence in lead free halide perovskite Cs3Bi2Br9: effect of structural distortion [J]. The Journal of Physical Chemistry C, 2021, 125(6): 3432–3440. doi: 10.1021/acs.jpcc.0c10624 [79] SHEN P F, VOGT T, LEE Y. Pressure-induced enhancement of broad-band white light emission in butylammonium lead bromide [J]. The Journal of Physical Chemistry Letters, 2020, 11(10): 4131–4137. doi: 10.1021/acs.jpclett.0c01160 [80] SHERWOOD B, RIDLEY C J, BULL C L, et al. A pressure induced reversal to the 9R perovskite in Ba3MoNbO8.5 [J]. Journal of Materials Chemistry A, 2021, 9(10): 6567–6574. doi: 10.1039/D0TA11270D [81] SHI Y, JIN Z Q, LV P F, et al. Bandgap narrowing and piezochromism of doped two-dimensional hybrid perovskite nanocrystals under pressure [J]. Journal of Materials Chemistry C, 2023, 11(5): 1726–1732. doi: 10.1039/D2TC05158C [82] SHI Y, ZHAO W Y, MA Z W, et al. Self-trapped exciton emission and piezochromism in conventional 3D lead bromide perovskite nanocrystals under high pressure [J]. Chemical Science, 2021, 12(44): 14711–14717. doi: 10.1039/D1SC04987A [83] SONG C P, YANG H R, LIU F, et al. Ultrafast femtosecond pressure modulation of structure and exciton kinetics in 2D halide perovskites for enhanced light response and stability [J]. Nature Communications, 2021, 12(1): 4879. doi: 10.1038/s41467-021-25140-2 [84] SUN M E, GENG T, YONG X, et al. Pressure-triggered blue emission of zero-dimensional organic bismuth bromide perovskite [J]. Advanced Science, 2021, 8(9): 2004853. doi: 10.1002/advs.202004853 [85] SUN M E, WANG Y G, WANG F, et al. Chirality-dependent structural transformation in chiral 2D perovskites under high pressure [J]. Journal of the American Chemical Society, 2023, 145(16): 8908–8916. doi: 10.1021/jacs.2c12527 [86] SUN S J, DENG Z Y, WU Y, et al. Variable temperature and high-pressure crystal chemistry of perovskite formamidinium lead iodide: a single crystal X-ray diffraction and computational study [J]. Chemical Communications, 2017, 53(54): 7537–7540. doi: 10.1039/C7CC00995J [87] SZAFRAŃSKI M, KATRUSIAK A, STÅHL K. Time-dependent transformation routes of perovskites CsPbBr3 and CsPbCl3 under high pressure [J]. Journal of Materials Chemistry A, 2021, 9(17): 10769–10779. doi: 10.1039/D1TA01875B [88] TIAN C, LIANG Y F, CHEN W H, et al. Hydrogen-bond enhancement triggered structural evolution and band gap engineering of hybrid perovskite (C6H5CH2NH3)2PbI4 under high pressure [J]. Physical Chemistry Chemical Physics, 2020, 22(4): 1841–1846. doi: 10.1039/C9CP05904K [89] WANG J X, WANG L R, WANG F, et al. Pressure-induced bandgap engineering of lead-free halide double perovskite (NH4)2SnBr6 [J]. Physical Chemistry Chemical Physics, 2021, 23(35): 19308–19312. doi: 10.1039/D1CP03267D [90] WANG L R, WANG K, ZOU B. Pressure-induced structural and optical properties of organometal halide perovskite-based formamidinium lead bromide [J]. The Journal of Physical Chemistry Letters, 2016, 7(13): 2556–2562. doi: 10.1021/acs.jpclett.6b00999 [91] WANG Y Q, GUO S H, LUO H, et al. Reaching 90% photoluminescence quantum yield in one-dimensional metal halide C4N2H14PbBr4 by pressure-suppressed nonradiative loss [J]. Journal of the American Chemical Society, 2020, 142(37): 16001–16006. doi: 10.1021/jacs.0c07166 [92] WANG Y J, ZHANG L K, MA S L, et al. Octahedral tilting dominated phase transition in compressed double perovskite Ba2SmBiO6 [J]. Applied Physics Letters, 2021, 118(23): 231903. doi: 10.1063/5.0054742 [93] WU L W, DONG Z Y, ZHANG L, et al. High-pressure band-gap engineering and metallization in the perovskite derivative Cs3Sb2I9 [J]. ChemSusChem, 2019, 12(17): 3971–3976. doi: 10.1002/cssc.201901388 [94] XIANG G B, WU Y W, ZHANG M, et al. Dimension-dependent bandgap narrowing and metallization in lead-free halide perovskite Cs3Bi2X9 (X = I, Br, and Cl) under high pressure [J]. Nanomaterials, 2021, 11(10): 2712. doi: 10.3390/nano11102712 [95] YANG H J, SHI W W, NAGAOKA Y, et al. Access and capture of layered double perovskite polytypic phase through high-pressure engineering [J]. The Journal of Physical Chemistry C, 2023, 127(5): 2407–2415. doi: 10.1021/acs.jpcc.2c07970 [96] ZHANG L, FANG Y Y, SUI L Z, et al. Tuning emission and electron-phonon coupling in lead-free halide double perovskite Cs2AgBiCl6 under pressure [J]. ACS Energy Letters, 2019, 4(12): 2975–2982. doi: 10.1021/acsenergylett.9b02155 [97] ZHANG L, LIU C M, WANG L R, et al. Pressure-induced emission enhancement, band-gap narrowing, and metallization of halide perovskite Cs3Bi2I9 [J]. Angewan Chemie International Edition, 2018, 57(35): 11213–11217. doi: 10.1002/anie.201804310 [98] ZHANG L, ZENG Q X, WANG K. Pressure-induced structural and optical properties of inorganic halide perovskite CsPbBr3 [J]. The Journal of Physical Chemistry Letters, 2017, 8(16): 3752–3758. doi: 10.1021/acs.jpclett.7b01577 [99] LI Q, WANG Y G, PAN W C, et al. High-pressure band-gap engineering in lead-free Cs2AgBiBr6 double perovskite [J]. Angewandte Chemie International Edition, 2017, 56(50): 15969–15973. doi: 10.1002/anie.201708684 [100] SHARMA S M, SIKKA S K. Pressure induced amorphization of materials [J]. Progress in Materials Science, 1996, 40(1): 1–77. doi: 10.1016/0079-6425(95)00006-2 [101] SHOCKLEY W, QUEISSER H J. Detailed balance limit of efficiency of p-n junction solar cells [J]. Journal of Applied Physics, 1961, 32(3): 510–519. doi: 10.1063/1.1736034 [102] LI M, LIU T B, WANG Y G, et al. Pressure responses of halide perovskites with various compositions, dimensionalities, and morphologies [J]. Matter and Radiation at Extremes, 2020, 5(1): 018201. doi: 10.1063/1.5133653 [103] ZHAO W J, RIBEIRO R M, EDA G. Electronic structure and optical signatures of semiconducting transition metal dichalcogenide nanosheets [J]. Accounts of Chemical Research, 2015, 48(1): 91–99. doi: 10.1021/ar500303m [104] TAUC J, GRIGOROVICI R, VANCU A. Optical properties and electronic structure of amorphous germanium [J]. Physica Status Solidi (b), 1966, 15(2): 627–637. doi: 10.1002/pssb.19660150224 [105] GAO C F, LI R P, LI Y R, et al. Direct-indirect transition of pressurized two-dimensional halide perovskite: role of benzene ring stack ordering [J]. The Journal of Physical Chemistry Letters, 2019, 10(19): 5687–5693. doi: 10.1021/acs.jpclett.9b02604 [106] GONG J B, ZHONG H X, GAO C, et al. Pressure-induced indirect-direct bandgap transition of CsPbBr3 single crystal and its effect on photoluminescence quantum yield [J]. Advanced Science, 2022, 9(29): 2201554. doi: 10.1002/advs.202201554 [107] WANG J X, WANG L R, LI Y Q, et al. Pressure-induced metallization of lead-free halide double perovskite (NH4)2PtI6 [J]. Advanced Science, 2022, 9(28): 2203442. doi: 10.1002/advs.202203442 [108] MATSUISHI K, ISHIHARA T, ONARI S, et al. Optical properties and structural phase transitions of lead-halide based inorganic-organic 3D and 2D perovskite semiconductors under high pressure [J]. Physica Status Solidi (B), 2004, 241(14): 3328–3333. doi: 10.1002/pssb.200405229 [109] YIN T T, FANG Y N, CHONG W K, et al. High-pressure-induced comminution and recrystallization of CH3NH3PbBr3 nanocrystals as large thin nanoplates [J]. Advanced Materials, 2018, 30(2): 1705017. doi: 10.1002/adma.201705017 [110] YIN T T, LIU B, YAN J X, et al. Pressure-engineered structural and optical properties of two-dimensional (C4H9NH3)2PbI4 perovskite exfoliated nm-thin flakes [J]. Journal of the American Chemical Society, 2019, 141(3): 1235–1241. doi: 10.1021/jacs.8b07765 [111] LIU S, SUN S S, GAN C K, et al. Manipulating efficient light emission in two-dimensional perovskite crystals by pressure-induced anisotropic deformation [J]. Science Advances, 2019, 5(7): eaav9445. doi: 10.1126/sciadv.aav9445 [112] GUO S H, ZHAO Y S, BU K J, et al. Pressure-suppressed carrier trapping leads to enhanced emission in two-dimensional perovskite (HA)2(GA)Pb2I7 [J]. Angewandte Chemie International Edition, 2020, 59(40): 17533–17539. doi: 10.1002/anie.202001635 [113] MA Z W, LI F F, SUI L Z, et al. Tunable color temperatures and emission enhancement in 1D halide perovskites under high pressure [J]. Advanced Optical Materials, 2020, 8(18): 2000713. doi: 10.1002/adom.202000713 [114] SHI Y, MA Z W, ZHAO D L, et al. Pressure-induced emission (PIE) of one-dimensional organic tin bromide perovskites [J]. Journal of the American Chemical Society, 2019, 141(16): 6504–6508. doi: 10.1021/jacs.9b02568 [115] REN X T, YAN X Z, AHMAD A S, et al. Pressure-induced phase transition and band gap engineering in propylammonium lead bromide perovskite [J]. The Journal of Physical Chemistry C, 2019, 123(24): 15204–15208. doi: 10.1021/acs.jpcc.9b02854 [116] LI Q, CHEN Z W, YANG B, et al. Pressure-induced remarkable enhancement of self-trapped exciton emission in one-dimensional CsCu2I3 with tetrahedral units [J]. Journal of the American Chemical Society, 2020, 142(4): 1786–1791. doi: 10.1021/jacs.9b13419 [117] LI Q, CHEN Z W, LI M Z, et al. Pressure-engineered photoluminescence tuning in zero-dimensional lead bromide trimer clusters [J]. Angewandte Chemie International Edition, 2021, 60(5): 2583–2587. doi: 10.1002/anie.202009237 [118] ZHANG L, LIU C M, LIN Y, et al. Tuning optical and electronic properties in low-toxicity organic-inorganic hybrid (CH3NH3)3Bi2I9 under high pressure [J]. The Journal of Physical Chemistry Letters, 2019, 10(8): 1676–1683. doi: 10.1021/acs.jpclett.9b00595 [119] MA Z W, LIU Z, LU S Y, et al. Pressure-induced emission of cesium lead halide perovskite nanocrystals [J]. Nature Communications, 2018, 9(1): 4506. doi: 10.1038/s41467-018-06840-8 [120] WANG T Y, DAIBER B, FROST J M, et al. Indirect to direct bandgap transition in methylammonium lead halide perovskite [J]. Energy & Environmental Science, 2017, 10(2): 509–515. doi: 10.1039/c6ee03474h [121] HAN Q F, BAE S H, SUN P Y, et al. Single crystal formamidinium lead iodide (FAPbI3): insight into the structural, optical, and electrical properties [J]. Advanced Materials, 2016, 28(11): 2253–2258. doi: 10.1002/adma.201505002 [122] MIZUSAKI J, ARAI K, FUEKI K. Ionic conduction of the perovskite-type halides [J]. Solid State Ionics, 1983, 11(3): 203–211. doi: 10.1016/0167-2738(83)90025-5 [123] YAMADA K, ISOBE K, TSUYAMA E, et al. Chloride ion conductor CH3NH3GeCl3 studied by Rietveld analysis of X-ray diffraction and 35Cl NMR [J]. Solid State Ionics, 1995, 79: 152–157. doi: 10.1016/0167-2738(95)00055-B [124] YAMADA K, KURANAGA Y, UEDA K, et al. Phase transition and electric conductivity of ASnCl3 (A= Cs and CH3NH3) [J]. Bulletin of the Chemical Society of Japan, 1998, 71(1): 127–134. doi: 10.1246/bcsj.71.127 [125] AZPIROZ J M, MOSCONI E, BISQUERT J, et al. Defect migration in methylammonium lead iodide and its role in perovskite solar cell operation [J]. Energy & Environmental Science, 2015, 8(7): 2118–2127. doi: 10.1039/c5ee01265a [126] YAN H C, OU T J, JIAO H, et al. Pressure dependence of mixed conduction and photo responsiveness in organolead tribromide perovskites [J]. The Journal of Physical Chemistry Letters, 2017, 8(13): 2944–2950. doi: 10.1021/acs.jpclett.7b01022 [127] LIN J, CHEN H, GAO Y, et al. Pressure-induced semiconductor-to-metal phase transition of a charge-ordered indium halide perovskite [J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(47): 23404–23409. doi: 10.1073/pnas.1907576116 [128] YAO X D, BAI Y X, JIN C, et al. Anomalous polarization enhancement in a van der Waals ferroelectric material under pressure [J]. Nature Communications, 2023, 14(1): 4301. doi: 10.1038/s41467-023-40075-6 -






 下载:
下载: