Research Progress of High Energy Density Nitrogen
-
摘要: 氮在常压下是非常稳定的元素,以氮气分子形式存在。研究发现,氮在高温高压下能够形成聚合结构,这种结构具有极高的能量密度,而且分解产物为无污染的氮气,从应用角度上看,它能够作为新型环保高能量密度材料。随后,人们对其进行了大量的研究,得到了氮在高压条件下的相图,并且合成出立方偏转氮、层状聚合氮等结构。然而,纯氮聚合结构的合成条件比较严苛,在常压下很难保存。人们又转向分子结构氮和惰性气体氮化物等,希望能够得到常压下稳定的高能量密度氮结构。为此,针对目前高能量密度氮的理论和实验进展进行了简要的介绍,并对未来高能量密度氮的发展方向进行了探讨。Abstract: Nitrogen is a highly stable element that exists in the form of nitrogen molecules under ambient pressure. Researchers have found that nitrogen can form polymeric structures under high temperature and pressure, which have extremely high energy density and decompose into pollution-free nitrogen. From the perspective of application, it can be used as a new type of environmentally friendly high-energy-density material. Subsequently, a large number of studies have been conducted on nitrogen, resulting in phase diagrams of nitrogen under high-pressure conditions and the synthesis of structures such as cubic gauche nitrogen and layered polymeric nitrogen. However, the synthesis conditions for pure nitrogen polymeric structures are relatively harsh, and it is also difficult to preserve them under ambient pressure. People have turned to methods such as molecular nitrogen and inert gas nitrides in the hope of obtaining stable high-energy-density nitrogen structures under normal pressure. This article briefly introduces the current theoretical and experimental progress of high-energy-density nitrogen and discusses the future development direction of high-energy-density nitrogen.
-
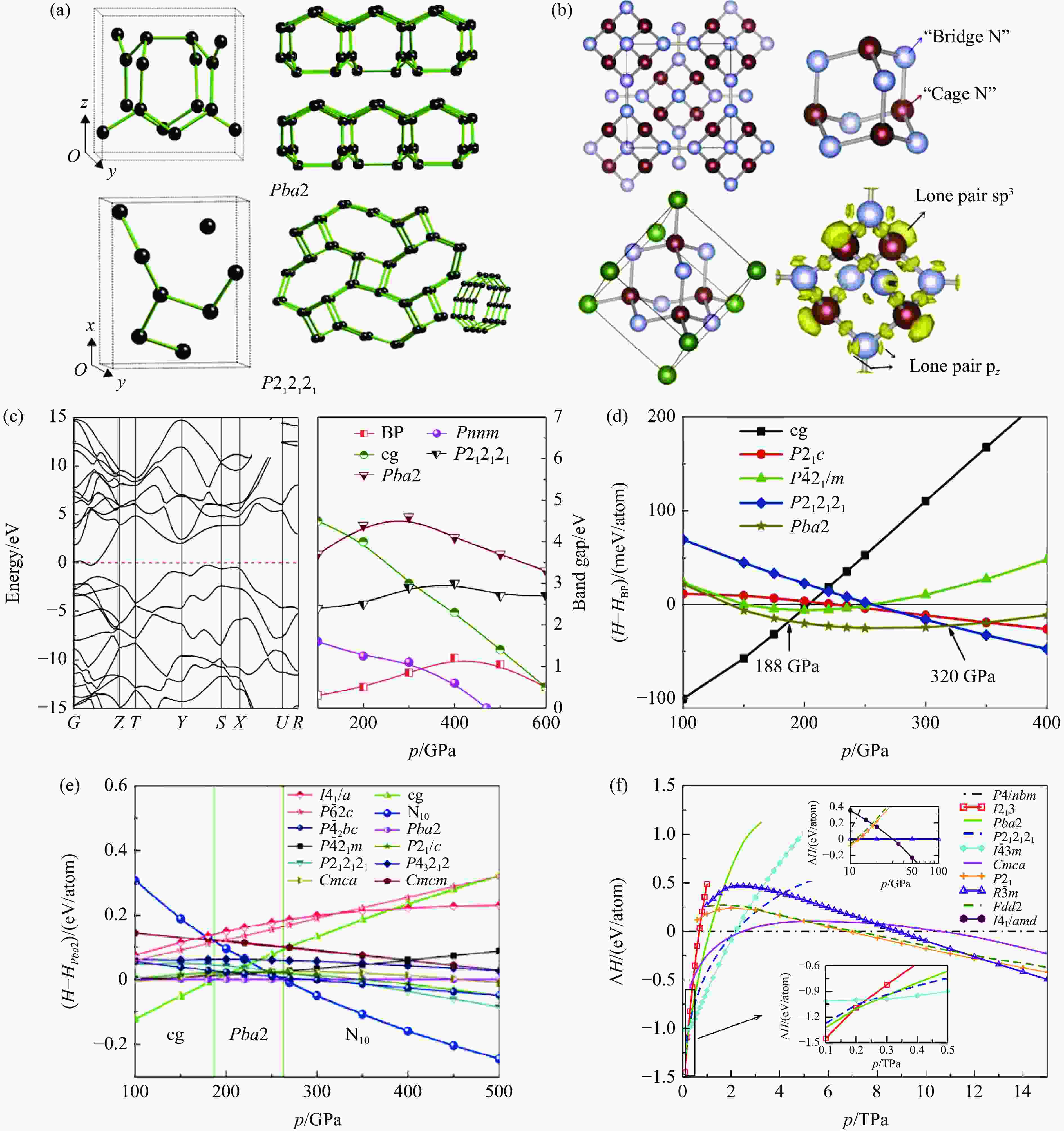
图 2 (a) Pba2和P212121的原胞和延伸结构[15],(b) N10的结构及ELF[20],(c) Pnnm相的能带结构和各种结构的带隙随压强的变化[21],(d) 100~400 GPa压强区间聚合氮的相图[15],(e) 100~500 GPa压强范围内聚合氮的相图[20],(f) 0~14 TPa压强范围内聚合氮的相图[22](H、HBP、HPba2分别为聚合氮、BP相和Pba2相的焓,ΔH为聚合氮与P4/nbm相的焓差)
Figure 2. (a) Primitive cell and extended structures of Pba2 and P212121[15]; (b) structure and ELF of N10[20]; (c) band structure of Pnnm phase and the band gap variation with pressure for various structures[21]; (d) phase diagram of polynitrogen from 100 GPa to 400 GPa[15]; (e) phase diagram of polynitrogen from 100 GPa to 500 GPa[20]; (f) phase diagram of polynitrogen from 0 TPato 14 TPa[22] (H, HBP, HPba2 represent the enthalpies of nitrogen polymeric phases, BP phase and Pba2 phase, respectively,and ΔH represents the enthalpy difference between various pure nitrogen polymeric phases and the P4/nbm phase.)
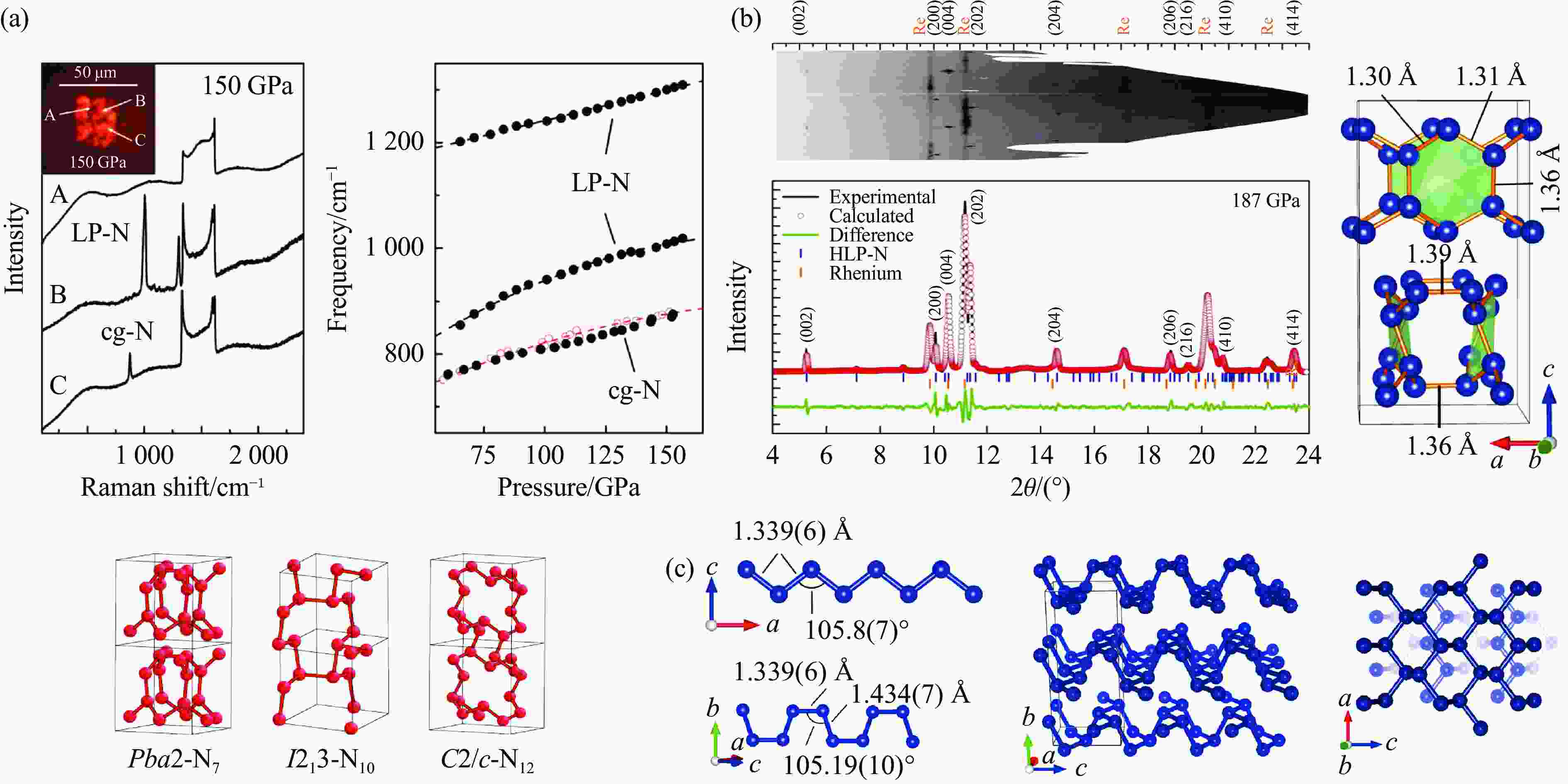
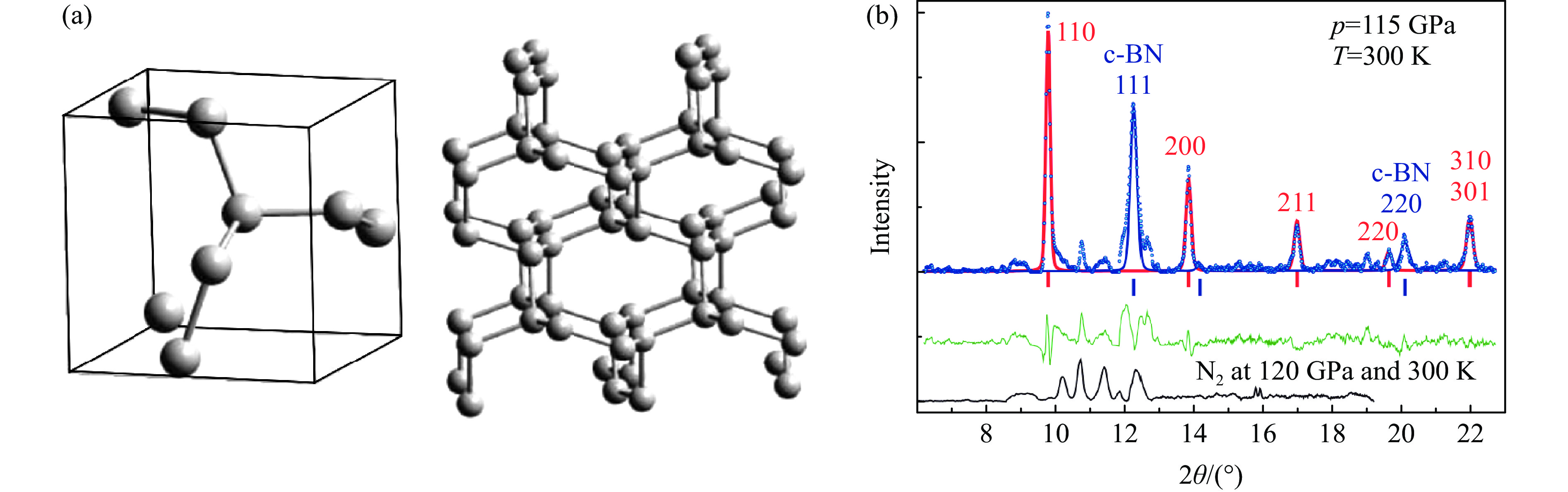
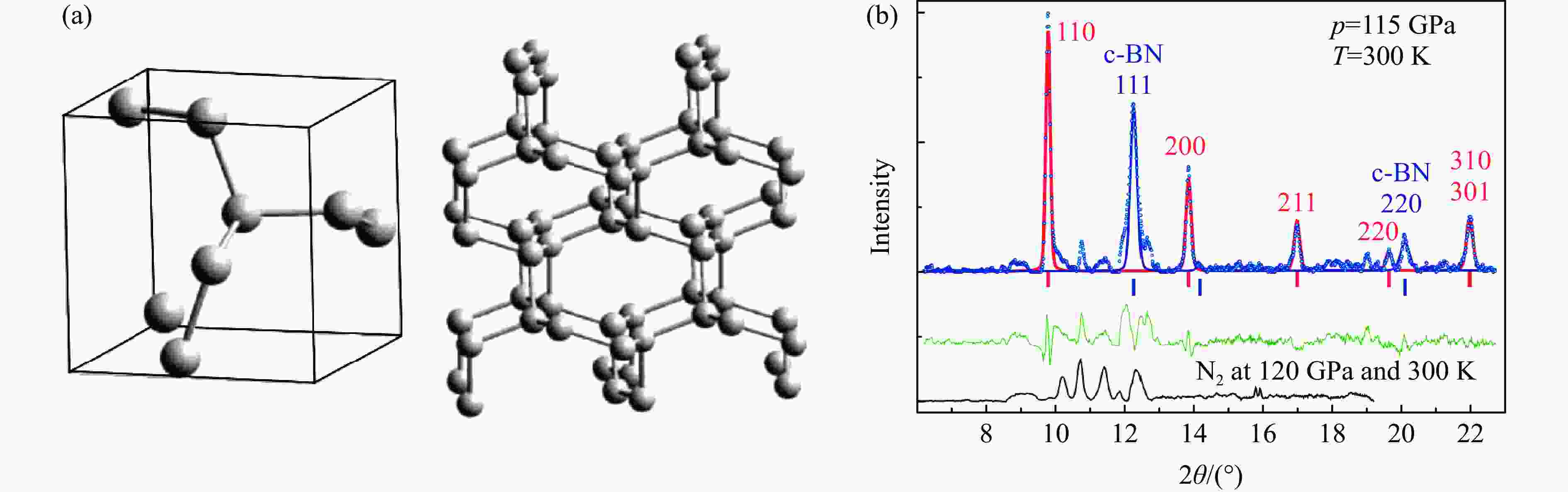
图 3 (a) 室温150 GPa下激光加热氮气的拉曼光谱、LP-N的2个特征振动频移随压力的变化与cg-N的对比、层状Pba2的晶体结构与三维结构的对比(左侧插图中,A、B、C分别代表黑色非晶态、LP-N、cg-N)[23],(b) HLP-N的X射线散射谱以及晶体结构[25],(c) BP-N的晶体结构[26]
Figure 3. (a) Raman spectra of laser-heated nitrogen at 150 GPa and ambient temperature, the pressure-dependent shifts of two characteristic vibration of LP-N shown in comparison with that of cg-N, and the comparison of the crystal structure and three-dimensional structure of layered Pba2 (A, B and C represent black amorphous, LP-N, and cg-N, respectively)[23]; (b) X-ray scattering spectrum and crystal structure of HLP-N[25]; (c) the crystal structure of BP-N[26]
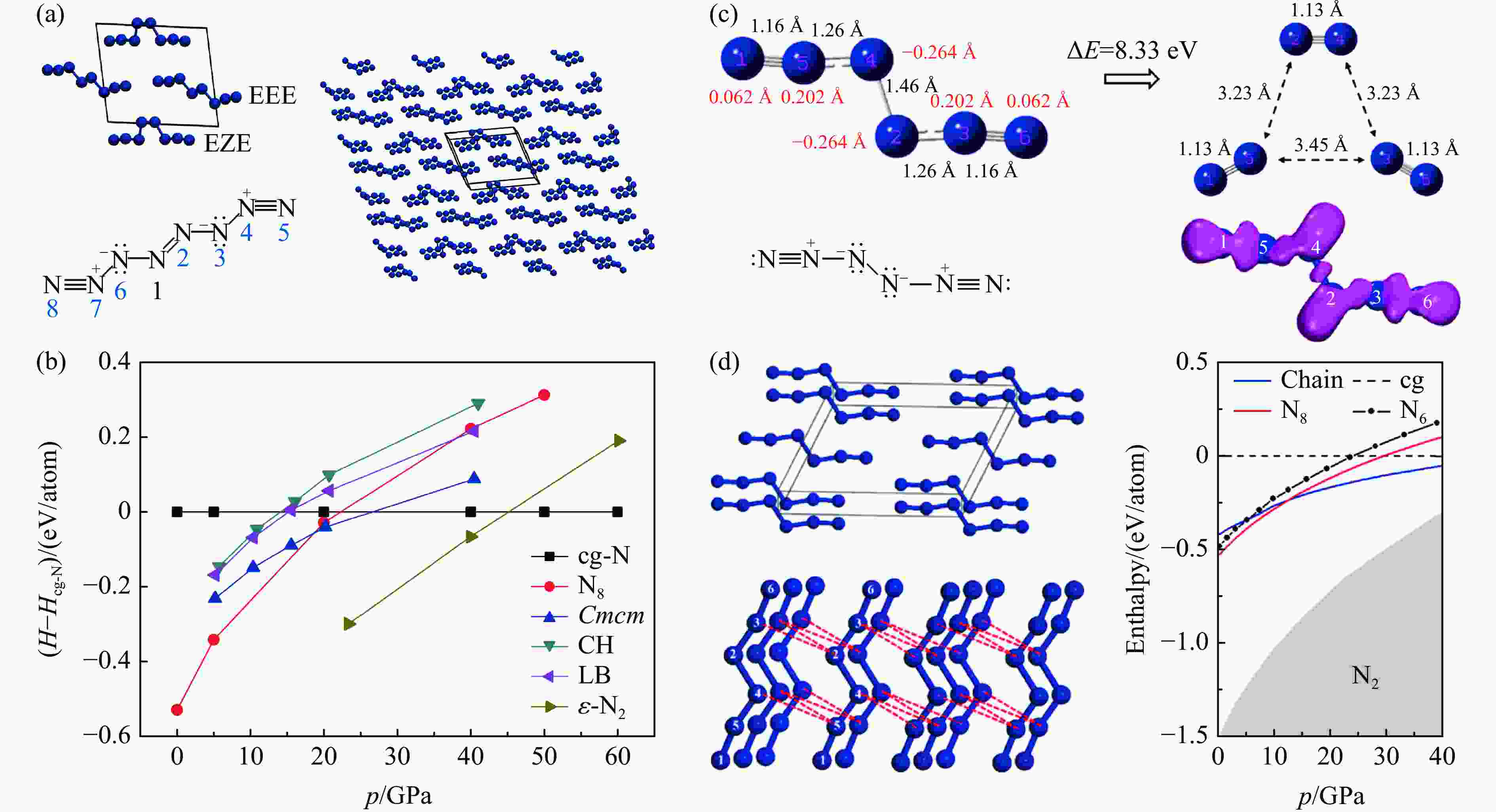
图 4 (a) N8的分子结构和晶体结构[34],(b) 0~60 GPa压强范围内各结构的焓[34],(c) N6链的电子结构[35],(d) N6分子组成的晶体结构及其焓[35]
Figure 4. (a) Structure and crystal structure of N8 molecule[34]; (b) enthalpy values of various structures from 0 GPa to 60 GPa[34];(c) electronic structure of N6 chain[35]; (d) crystal structure and enthalpy values of N6 molecule[35]
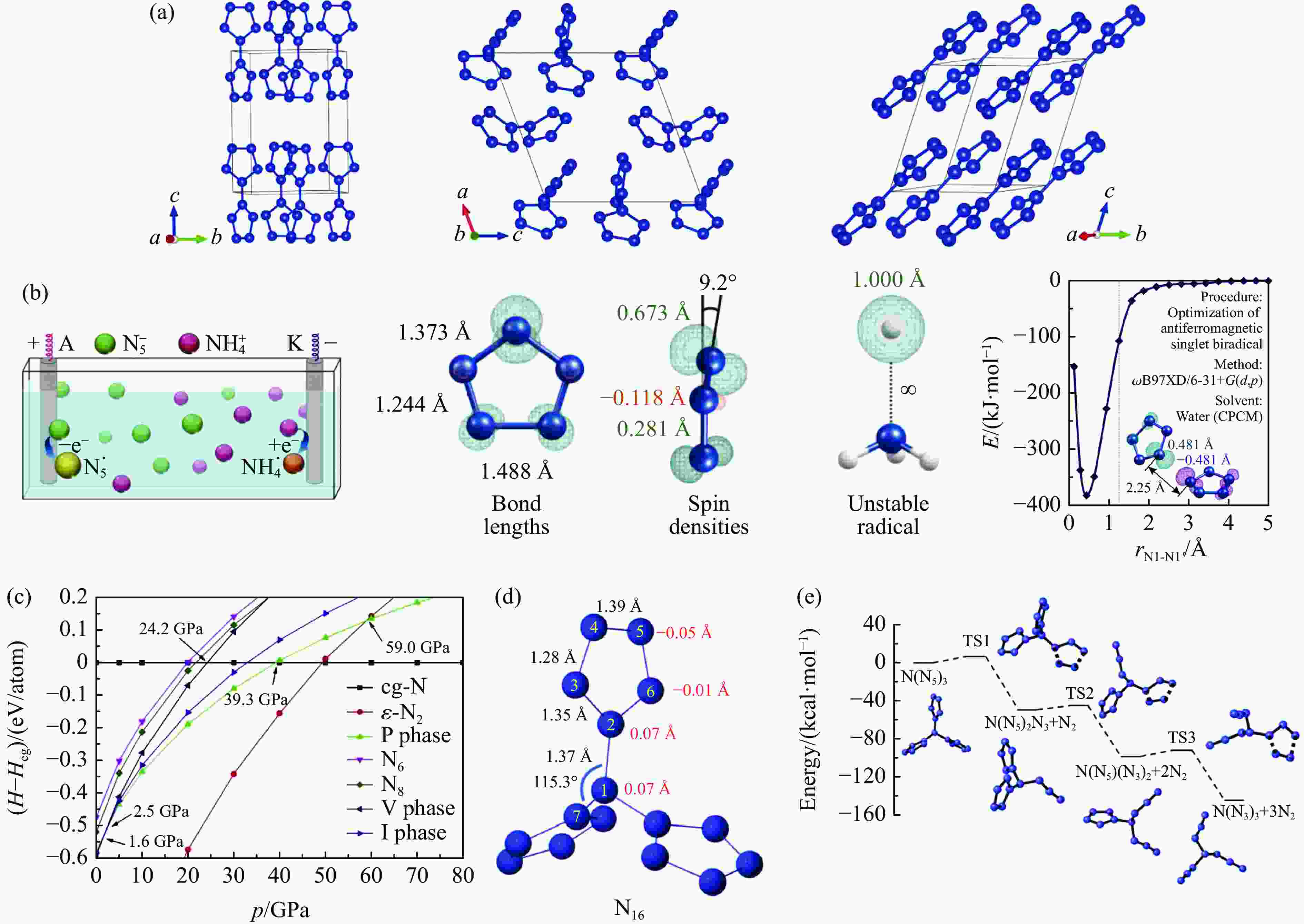
图 5 (a) V型、P型以及I型N10的晶体结构[38],(b) N10分子的可能合成路径[39],(c) 0~80 GPa压强区间的焓值[38],(d) N16的分子结构[40],(e) N16分子的分解路径[40]
Figure 5. (a) Crystal structures of N10 for V-type, P-type, and I-type[38]; (b) possible synthesis path of N10 molecule[39]; (c) enthalpy values from 0 GPa to 80 GPa[38]; (d) structure of N16 molecule[40]; (e) decomposition path of N16 molecule[40]
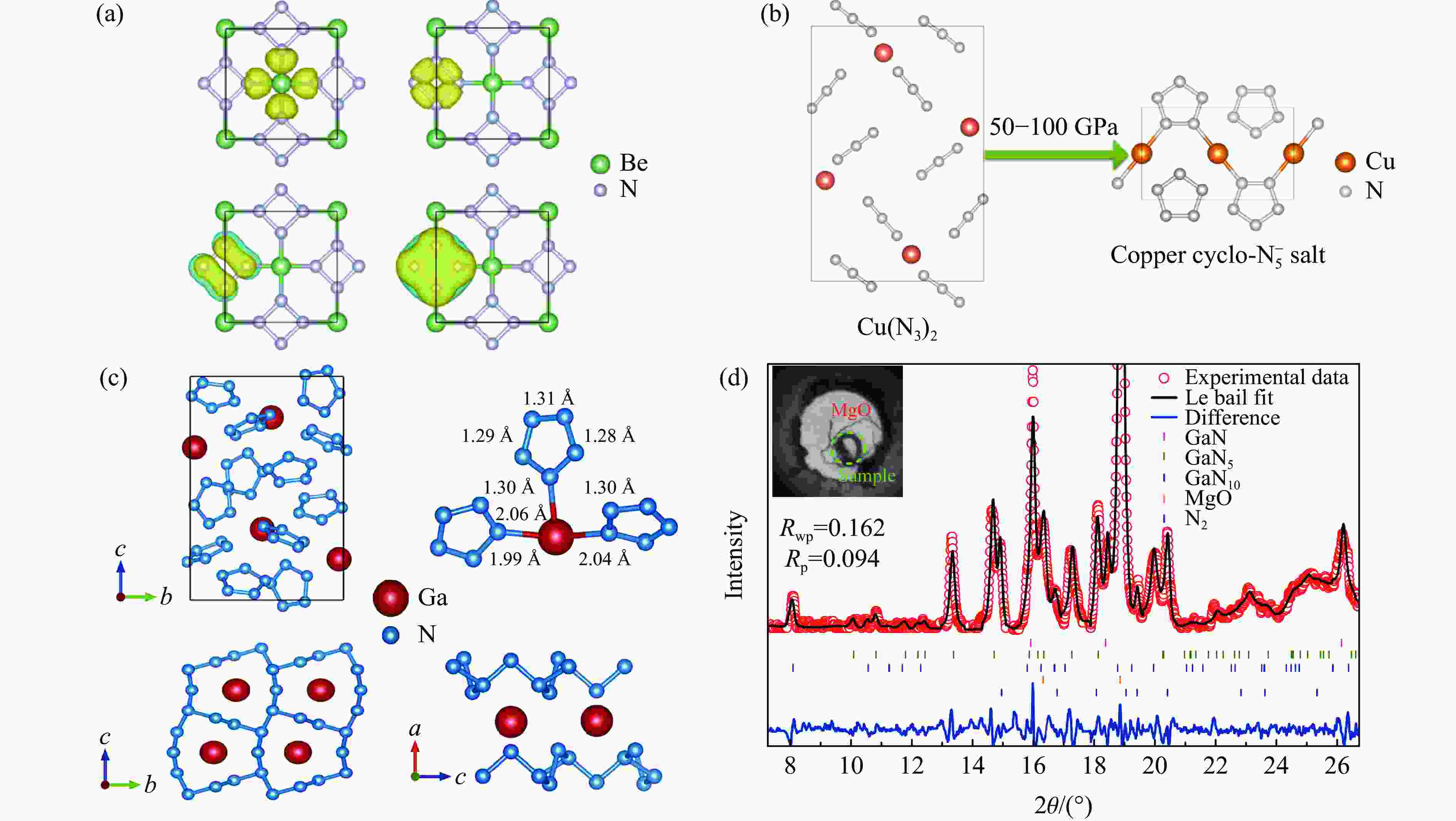
图 6 (a) P4/nmm-BeN4的成键模式[54],(b) P21/m-CuN5的可能合成路径[51],(c) Ga-N化合物的晶体结构[53],(d) 85 GPa下Ga-N化合物的XRD谱[53]
Figure 6. (a) Chemical bonding pattern of P4/nmm-BeN4[54]; (b) possible synthesis route of P21/m-CuN5[51]; (c) crystal structures of Ga-N compounds[53]; (d) XRD patterns of Ga-N compounds at 85 GPa[53]
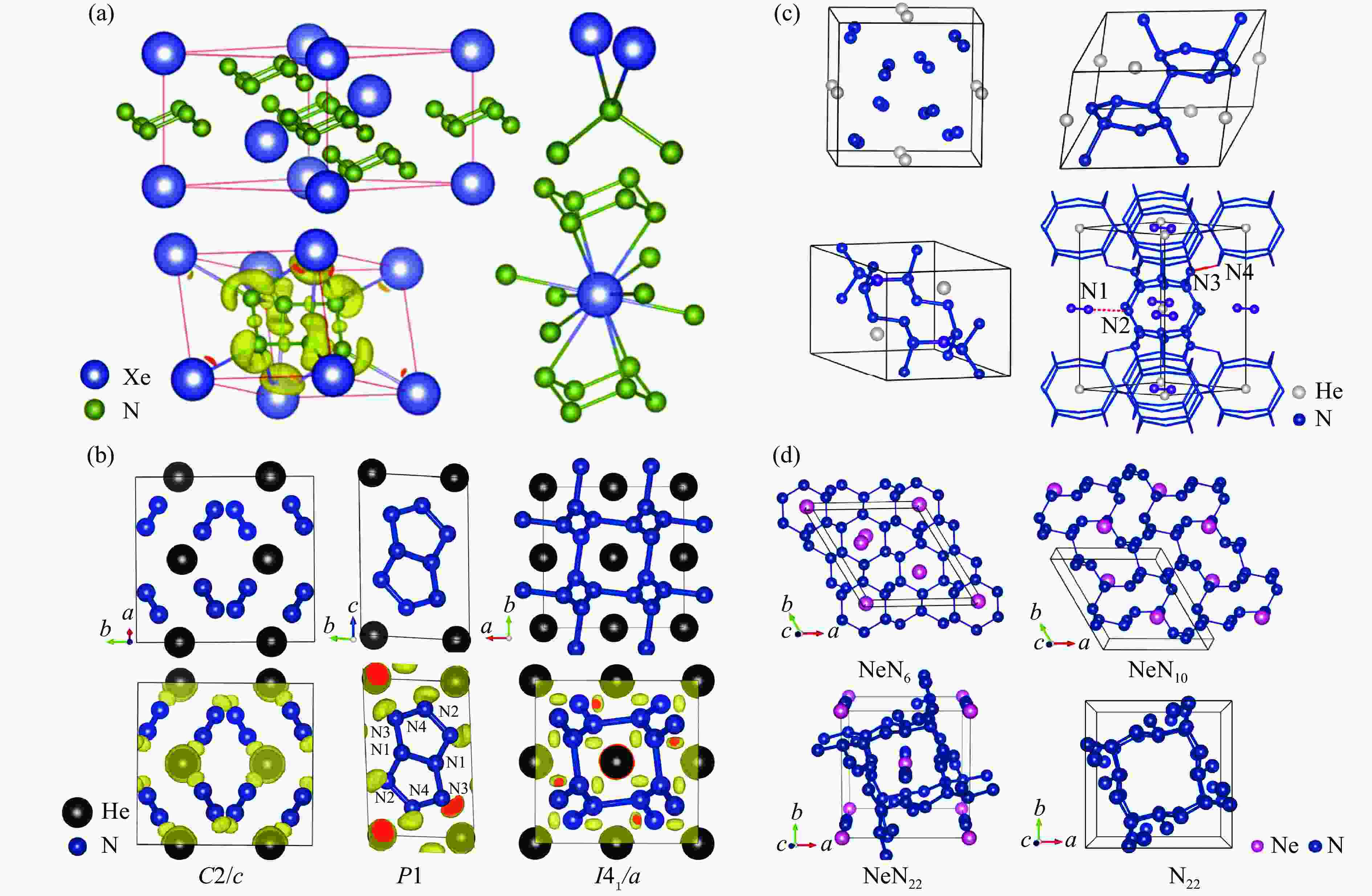
图 7 (a) XeN6的晶体结构和成键性质[55],(b) HeN4的晶体结构和成键性质[56],(c) He-N体系的晶体结构[57],(d) Ne-N体系的晶体结构以及纯氮框架[58]
Figure 7. (a) Crystal structure and bonding properties of XeN6[55]; (b) crystal structure and bonding properties of HeN4[56]; (c) crystal structure of He-N compounds[57]; (d) crystal structures of Ne-N compounds and pure nitrogen[58]
-
[1] MAILHIOT C, YANG L H, MCMAHAN A K. Polymeric nitrogen [J]. Physical Review B, 1992, 46(22): 14419–14435. doi: 10.1103/PhysRevB.46.14419 [2] MCMAHAN A K, LESAR R. Pressure dissociation of solid nitrogen under 1 Mbar [J]. Physical Review Letters, 1985, 54(17): 1929–1932. doi: 10.1103/PhysRevLett.54.1929 [3] MARTIN R M, NEEDS R J. Theoretical study of the molecular-to-nonmolecular transformation of nitrogen at high pressures [J]. Physical Review B, 1986, 34(8): 5082–5092. doi: 10.1103/PhysRevB.34.5082 [4] LEWIS S P, COHEN M L. High-pressure atomic phases of solid nitrogen [J]. Physical Review B, 1992, 46(17): 11117–11120. doi: 10.1103/PhysRevB.46.11117 [5] LI Q S, LIU Y D. Theoretical studies of the N6 potential energy surface [J]. The Journal of Physical Chemistry A, 2002, 106(41): 9538–9542. doi: 10.1021/jp0258917 [6] ALEMANY M M G, MARTINS J L. Density-functional study of nonmolecular phases of nitrogen: metastable phase at low pressure [J]. Physical Review B, 2003, 68(2): 024110. doi: 10.1103/PhysRevB.68.024110 [7] CARACAS R, HEMLEY R J. New structures of dense nitrogen: pathways to the polymeric phase [J]. Chemical Physics Letters, 2007, 442(1/2/3): 65–70. doi: 10.1016/j.cplett.2007.05.053 [8] EREMETS M I, GAVRILIUK A G, TROJAN I A, et al. Single-bonded cubic form of nitrogen [J]. Nature Materials, 2004, 3(8): 558–563. doi: 10.1038/nmat1146 [9] EREMETS M I, GAVRILIUK A G, SEREBRYANAYA N R, et al. Structural transformation of molecular nitrogen to a single-bonded atomic state at high pressures [J]. The Journal of Chemical Physics, 2004, 121(22): 11296–11300. doi: 10.1063/1.1814074 [10] BENCHAFIA E M, YAO Z H, YUAN G, et al. Cubic gauche polymeric nitrogen under ambient conditions [J]. Nature Communications, 2017, 8(1): 930. [11] KAMLET M J, JACOBS S J. Chemistry of detonations. Ⅰ. a simple method for calculating detonation properties of C-H-N-O explosives [J]. The Journal of Chemical Physics, 1968, 48(1): 23−35. [12] HANG G Y, YU W L, WANG T, et al. Molecular dynamics calculation on structures, stabilities, mechanical properties, and energy density of CL-20/FOX-7 cocrystal explosives [J]. Journal of Molecular Modeling, 2017, 23(12): 362. doi: 10.1007/s00894-017-3533-3 [13] GONCHAROV A F, GREGORYANZ E, MAO H K, et al. Optical evidence for a nonmolecular phase of nitrogen above 150 GPa [J]. Physical Review Letters, 2000, 85(6): 1262–1265. doi: 10.1103/PhysRevLett.85.1262 [14] WANG X L, HE Z, MA Y M, et al. Prediction of a new layered phase of nitrogen from first-principles simulations [J]. Journal of Physics: Condensed Matter, 2007, 19(42): 425226. doi: 10.1088/0953-8984/19/42/425226 [15] MA Y M, OGANOV A R, LI Z W, et al. Novel high pressure structures of polymeric nitrogen [J]. Physical Review Letters, 2009, 102(6): 065501. doi: 10.1103/PhysRevLett.102.065501 [16] PICKARD C J, NEEDS R J. High-pressure phases of nitrogen [J]. Physical Review Letters, 2009, 102(12): 125702. doi: 10.1103/PhysRevLett.102.125702 [17] MATTSON W D, SANCHEZ-PORTAL D, CHIESA S, et al. Prediction of new phases of nitrogen at high pressure from first-principles simulations [J]. Physical Review Letters, 2004, 93(12): 125501. doi: 10.1103/PhysRevLett.93.125501 [18] ZAHARIEV F, HOOPER J, ALAVI S, et al. Low-pressure metastable phase of single-bonded polymeric nitrogen from a helical structure motif and first-principles calculations [J]. Physical Review B, 2007, 75(14): 140101. doi: 10.1103/PhysRevB.75.140101 [19] WANG X L, TIAN F B, WANG L C, et al. Structural stability of polymeric nitrogen: a first-principles investigation [J]. The Journal of Chemical Physics, 2010, 132(2): 024502. doi: 10.1063/1.3290954 [20] WANG X L, WANG Y C, MIAO M S, et al. Cagelike diamondoid nitrogen at high pressures [J]. Physical Review Letters, 2012, 109(17): 175502. doi: 10.1103/PhysRevLett.109.175502 [21] WANG X L, TIAN F B, WANG L, et al. Predicted novel metallic metastable phases of polymeric nitrogen at high pressures [J]. New Journal of Physics, 2013, 15(1): 013010. doi: 10.1088/1367-2630/15/1/013010 [22] SUN J, MARTINEZ-CANALES M, KLUG D D, et al. Stable all-nitrogen metallic salt at terapascal pressures [J]. Physical Review Letters, 2013, 111(17): 175502. doi: 10.1103/PhysRevLett.111.175502 [23] TOMASINO D, KIM M, SMITH J, et al. Pressure-induced symmetry-lowering transition in dense nitrogen to layered polymeric nitrogen (LP-N) with colossal Raman intensity [J]. Physical Review Letters, 2014, 113(20): 205502. doi: 10.1103/PhysRevLett.113.205502 [24] ADELEKE A A, GRESCHNER M J, MAJUMDAR A, et al. Single-bonded allotrope of nitrogen predicted at high pressure [J]. Physical Review B, 2017, 96(22): 224104. doi: 10.1103/PhysRevB.96.224104 [25] LANIEL D, GENESTE G, WECK G, et al. Hexagonal layered polymeric nitrogen phase synthesized near 250 GPa [J]. Physical Review Letters, 2019, 122(6): 066001. doi: 10.1103/PhysRevLett.122.066001 [26] LANIEL D, WINKLER B, FEDOTENKO T, et al. High-pressure polymeric nitrogen allotrope with the black phosphorus structure [J]. Physical Review Letters, 2020, 124(21): 216001. doi: 10.1103/PhysRevLett.124.216001 [27] JI C, ADELEKE A A, YANG L X, et al. Nitrogen in black phosphorus structure [J]. Science Advances, 2020, 6(23): eaba9206. doi: 10.1126/sciadv.aba9206 [28] LI Q S, ZHAO J F. Theoretical study of potential energy surfaces for N12 clusters [J]. The Journal of Physical Chemistry A, 2002, 106(21): 5367–5372. doi: 10.1021/jp020110n [29] STROUT D L. Cage isomers of N14 and N16 : nitrogen molecules that are not a multiple of six [J]. The Journal of Physical Chemistry A, 2004, 108(49): 10911–10916. doi: 10.1021/jp046496e [30] SAMARTZIS P C, WODTKE A M. All-nitrogen chemistry: how far are we from N60? [J]. International Reviews in Physical Chemistry, 2006, 25(4): 527–552. doi: 10.1080/01442350600879319 [31] REN Y, WANG X, WONG N B, et al. Theoretical study of the N10 clusters without double bonds [J]. International Journal of Quantum Chemistry, 2001, 82(1): 34–43. doi: 10.1002/1097-461X(2001)82:1<34::AID-QUA1013>3.0.CO;2-1 [32] GUAN J, ZHANG S W, XU W G, et al. A quantum chemical study of N14 cluster [J]. Structural Chemistry, 2004, 15(2): 121–132. doi: 10.1023/B:STUC.0000011247.54952.89 [33] HA T K, SULEIMENOV O, NGUYEN M T. A quantum chemical study of three isomers of N20 [J]. Chemical Physics Letters, 1999, 315(5/6): 327–334. doi: 10.1016/S0009-2614(99)01271-3 [34] HIRSHBERG B, GERBER R B, KRYLOV A I. Calculations predict a stable molecular crystal of N8 [J]. Nature Chemistry, 2014, 6(1): 52–56. doi: 10.1038/nchem.1818 [35] GRESCHNER M J, ZHANG M, MAJUMDAR A, et al. A new allotrope of nitrogen as high-energy density material [J]. The Journal of Physical Chemistry A, 2016, 120(18): 2920–2925. doi: 10.1021/acs.jpca.6b01655 [36] ZHANG C, SUN C G, HU B C, et al. Synthesis and characterization of the pentazolate anion cyclo- $ {\rm N_5^-} $ in (N5)6(H3O)3(NH4)4Cl [J]. Science, 2017, 355(6323): 374–376. doi: 10.1126/science.aah3840[37] XU Y G, WANG Q, SHEN C, et al. A series of energetic metal pentazolate hydrates [J]. Nature, 2017, 549(7670): 78–81. doi: 10.1038/nature23662 [38] LIU S J, ZHAO L, YAO M G, et al. Novel all-nitrogen molecular crystals of aromatic N10 [J]. Advanced Science, 2020, 7(10): 1902320. doi: 10.1002/advs.201902320 [39] BONDARCHUK S V. Bipentazole (N10): a low-energy molecular nitrogen allotrope with high intrinsic stability [J]. The Journal of Physical Chemistry Letters, 2020, 11(14): 5544–5548. doi: 10.1021/acs.jpclett.0c01542 [40] ZHAO L, LIU S J, CHEN Y Z, et al. A novel all-nitrogen molecular crystal N16 as a promising high-energy-density material [J]. Dalton Transactions, 2022, 51(24): 9369–9376. doi: 10.1039/D2DT00820C [41] YUAN J N, XIA K, DING C, et al. High-energy-density metal nitrides with armchair chains [J]. Matter and Radiation at Extremes, 2022, 7(3): 038402. doi: 10.1063/5.0087168 [42] LI F, WANG Y, WU H, et al. Benzene-like N6 rings in a Be2N6 monolayer: a stable 2D semiconductor with high carrier mobility [J]. Journal of Materials Chemistry C, 2017, 5(44): 11515–11521. doi: 10.1039/C7TC03363J [43] LIU Z, LI D, WEI S L, et al. Bonding properties of aluminum nitride at high pressure [J]. Inorganic Chemistry, 2017, 56(13): 7494–7500. doi: 10.1021/acs.inorgchem.7b00980 [44] PENG F, YAO Y S, LIU H Y, et al. Crystalline LiN5 predicted from first-principles as a possible high-energy material [J]. The Journal of Physical Chemistry Letters, 2015, 6(12): 2363–2366. doi: 10.1021/acs.jpclett.5b00995 [45] BYKOV M, BYKOVA E, APRILIS G, et al. Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains [J]. Nature Communications, 2018, 9(1): 2756. doi: 10.1038/s41467-018-05143-2 [46] XIA K, YUAN J N, ZHENG X X, et al. Predictions on high-power trivalent metal pentazolate salts [J]. The Journal of Physical Chemistry Letters, 2019, 10(20): 6166–6173. doi: 10.1021/acs.jpclett.9b02383 [47] YUAN J N, XIA K, WU J F, et al. High-energy-density pentazolate salts: CaN10 and BaN10 [J]. Science China Physics, Mechanics & Astronomy, 2021, 64(1): 218211. [48] YUAN J N, CHI D, COGOLLO-OLIVO B H, et al. Prediction of novel tetravalent metal pentazolate salts with anharmonic effect [J]. Fundamental Research, 2022. DOI: 10.1016/j.fmre.2022.10.017. [49] XIA K, GAO H, LIU C, et al. A novel superhard tungsten nitride predicted by machine-learning accelerated crystal structure search [J]. Science Bulletin, 2018, 63(13): 817–824. doi: 10.1016/j.scib.2018.05.027 [50] XIA K, ZHENG X X, YUAN J N, et al. Pressure-stabilized high-energy-density alkaline-earth-metal pentazolate salts [J]. The Journal of Physical Chemistry C, 2019, 123(16): 10205–10211. doi: 10.1021/acs.jpcc.8b12527 [51] LI J F, SUN L, WANG X L, et al. Simple route to metal cyclo-N5-salt: high-pressure synthesis of CuN5 [J]. The Journal of Physical Chemistry C, 2018, 122(39): 22339–22344. doi: 10.1021/acs.jpcc.8b08924 [52] LIN J N, PENG D, WANG Q L, et al. Stable nitrogen-rich scandium nitrides and their bonding features under ambient conditions [J]. Physical Chemistry Chemical Physics, 2021, 23(11): 6863–6870. doi: 10.1039/D0CP05402J [53] ZHAI H, XU R, DAI J H, et al. Stabilized nitrogen framework anions in the Ga-N system [J]. Journal of the American Chemical Society, 2022, 144(47): 21640–21647. doi: 10.1021/jacs.2c09056 [54] LIN J N, WANG F X, RUI Q, et al. A novel square planar N42-ring with aromaticity in BeN4 [J]. Matter and Radiation at Extremes, 2022, 7(3): 038401. doi: 10.1063/5.0084802 [55] PENG F, WANG Y C, WANG H, et al. Stable xenon nitride at high pressures [J]. Physical Review B, 2015, 92(9): 094104. doi: 10.1103/PhysRevB.92.094104 [56] LI Y W, FENG X L, LIU H Y, et al. Route to high-energy density polymeric nitrogen t-N via He-N compounds [J]. Nature Communications, 2018, 9(1): 722. doi: 10.1038/s41467-018-03200-4 [57] HOU J Y, WENG X J, OGANOV A R, et al. Helium-nitrogen mixtures at high pressure [J]. Physical Review B, 2021, 103(6): L060102. doi: 10.1103/PhysRevB.103.L060102 [58] LIU L L, ZHANG S T, ZHANG H J. Pressure-driven Ne-bearing polynitrides with ultrahigh energy density [J]. Chinese Physics Letters, 2022, 39(5): 056102. doi: 10.1088/0256-307X/39/5/056102 -






 下载:
下载: