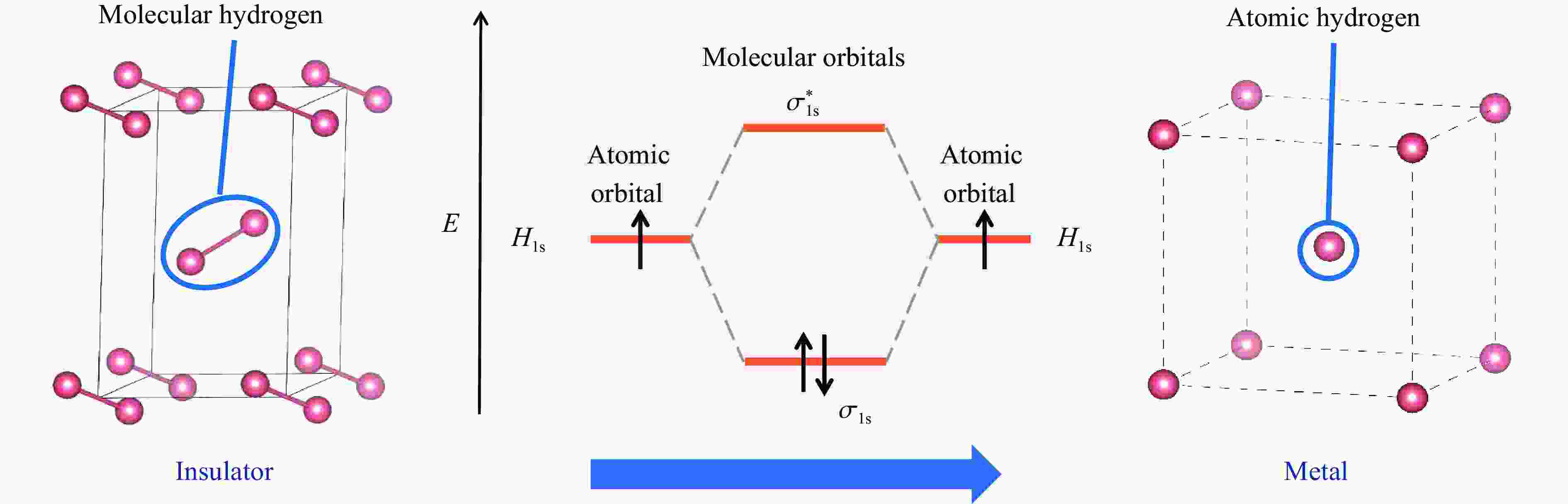
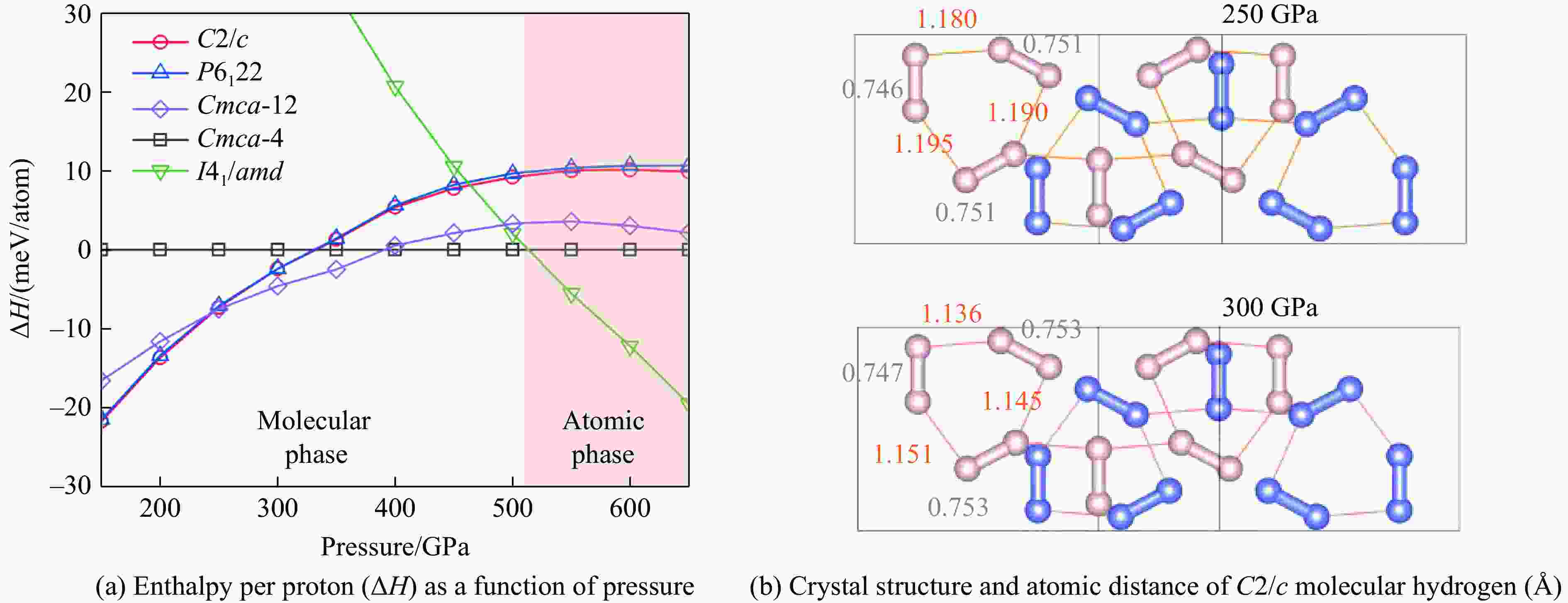
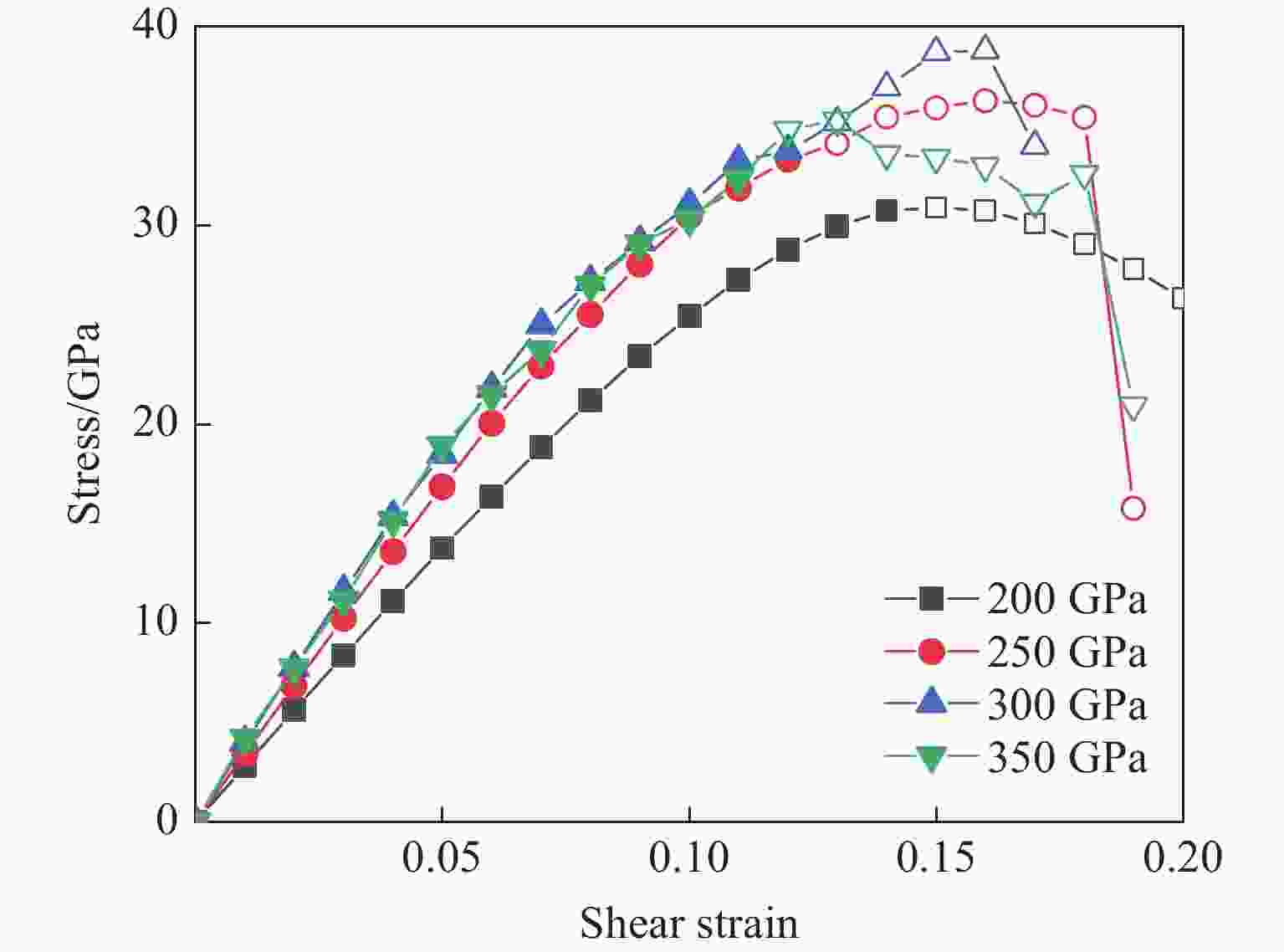
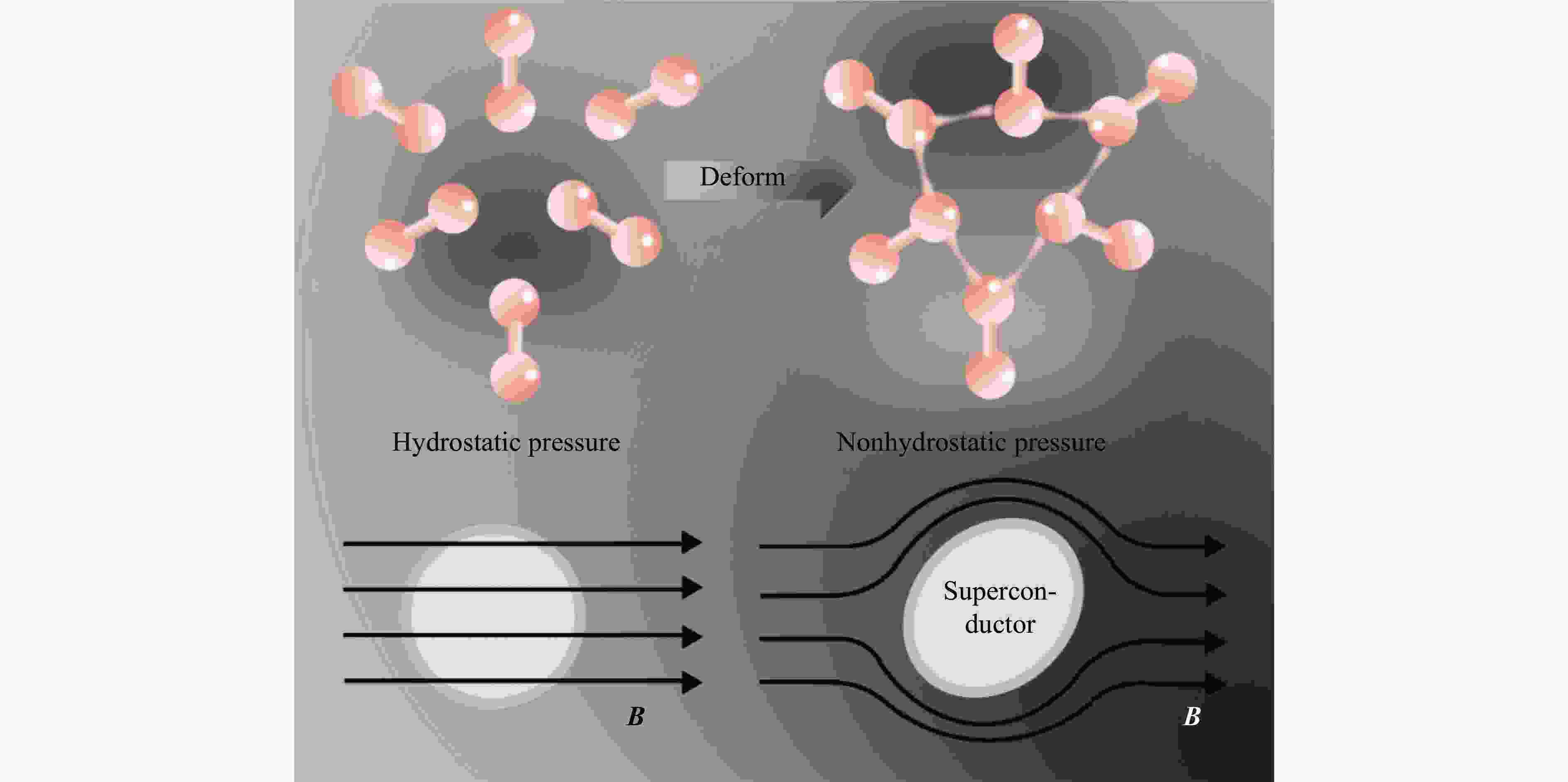
| [1] |
WIGNER E, HUNTINGTON H B. On the possibility of a metallic modification of hydrogen [J]. The Journal of Chemical Physics, 1935, 3(12): 764–770. doi: 10.1063/1.1749590
|
| [2] |
ASHCROFT N W. Metallic hydrogen: a high-temperature superconductor? [J]. Physical Review Letters, 1968, 21(26): 1748–1749. doi: 10.1103/PhysRevLett.21.1748
|
| [3] |
BALL P. Metallic hydrogen in the spotlight [J]. Nature Materials, 2017, 16(3): 288. doi: 10.1038/nmat4872
|
| [4] |
DIAS R P, SILVERA I F. Observation of the Wigner-Huntington transition to metallic hydrogen [J]. Science, 2017, 355(6326): 715–718. doi: 10.1126/science.aal1579
|
| [5] |
EREMETS M I, DROZDOV A P, KONG P P, et al. Semimetallic molecular hydrogen at pressure above 350 GPa [J]. Nature Physics, 2019, 15(12): 1246–1249. doi: 10.1038/s41567-019-0646-x
|
| [6] |
LOUBEYRE P, OCCELLI F, DUMAS P. Synchrotron infrared spectroscopic evidence of the probable transition to metal hydrogen [J]. Nature, 2020, 577(7792): 631–635. doi: 10.1038/s41586-019-1927-3
|
| [7] |
MONACELLI L, ERREA I, CALANDRA M, et al. Black metal hydrogen above 360 GPa driven by proton quantum fluctuations [J]. Nature Physics, 2021, 17(1): 63–67. doi: 10.1038/s41567-020-1009-3
|
| [8] |
SONG X Q, LIU C, LI Q, et al. Stress-induced high- Tc superconductivity in solid molecular hydrogen [J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(26): e2122691119. doi: 10.1073/PNAS.2122691119
|
| [9] |
ZHANG L J, WANG Y C, LV J, et al. Materials discovery at high pressures [J]. Nature Reviews Materials, 2017, 2(4): 17005. doi: 10.1038/natrevmats.2017.5
|
| [10] |
李全, 马琰铭. 典型双原子分子晶体的高压解离和单原子相[J]. 高压物理学报, 2013, 27(3): 313–324. doi: 10.11858/gywlxb.2013.03.001LI Q, MA Y M. High pressure dissociation of typical diatomic molecular solids and their atomic phases [J]. Chinese Journal of High Pressure Physics, 2013, 27(3): 313–324. doi: 10.11858/gywlxb.2013.03.001
|
| [11] |
DALLADAY-SIMPSON P, BINNS J, PEÑA-ALVAREZ M, et al. Band gap closure, incommensurability and molecular dissociation of dense chlorine [J]. Nature Communications, 2019, 10(1): 1134. doi: 10.1038/s41467-019-09108-x
|
| [12] |
EREMETS M I, GAVRILIUK A G, TROJAN I A, et al. Single-bonded cubic form of nitrogen [J]. Nature Materials, 2004, 3(8): 558–563. doi: 10.1038/nmat1146
|
| [13] |
WANG X L, WANG Y C, MIAO M S, et al. Cagelike diamondoid nitrogen at high pressures [J]. Physical Review Letters, 2012, 109(17): 175502. doi: 10.1103/PhysRevLett.109.175502
|
| [14] |
JI C, ADELEKE A A, YANG L X, et al. Nitrogen in black phosphorus structure [J]. Science Advances, 2020, 6(23): eaba9206. doi: 10.1126/sciadv.aba9206
|
| [15] |
DUAN D F, LIU Z T, LIN Z Y, et al. Multistep dissociation of fluorine molecules under extreme compression [J]. Physical Review Letters, 2021, 126(22): 225704. doi: 10.1103/PhysRevLett.126.225704
|
| [16] |
BARDEEN J, COOPER L N, SCHRIEFFER J R. Microscopic theory of superconductivity [J]. Physical Review, 1957, 106(1): 162–164. doi: 10.1103/PhysRev.106.162
|
| [17] |
BARDEEN J, COOPER L N, SCHRIEFFER J R. Theory of superconductivity [J]. Physical Review, 1957, 108(5): 1175–1204. doi: 10.1103/PhysRev.108.1175
|
| [18] |
PICKARD C J, NEEDS R J. Structure of phase Ⅲ of solid hydrogen [J]. Nature Physics, 2007, 3(7): 473–476. doi: 10.1038/nphys625
|
| [19] |
PICKARD C J, MARTINEZ-CANALES M, NEEDS R J. Density functional theory study of phase Ⅳ of solid hydrogen [J]. Physical Review B, 2012, 85(21): 214114. doi: 10.1103/PhysRevB.85.214114
|
| [20] |
MCMINIS J, CLAY III R C, LEE D, et al. Molecular to atomic phase transition in hydrogen under high pressure [J]. Physical Review Letters, 2015, 114(10): 105305. doi: 10.1103/PhysRevLett.114.105305
|
| [21] |
DALLADAY-SIMPSON P, HOWIE R T, GREGORYANZ E. Evidence for a new phase of dense hydrogen above 325 gigapascals [J]. Nature, 2016, 529(7584): 63–67. doi: 10.1038/nature16164
|
| [22] |
MONSERRAT B, DRUMMOND N D, DALLADAY-SIMPSON P, et al. Structure and metallicity of phase Ⅴ of hydrogen [J]. Physical Review Letters, 2018, 120(25): 255701. doi: 10.1103/PhysRevLett.120.255701
|
| [23] |
MAO H K, HEMLEY R J. Ultrahigh-pressure transitions in solid hydrogen [J]. Reviews of Modern Physics, 1994, 66(2): 671–692. doi: 10.1103/RevModPhys.66.671
|
| [24] |
LORENZANA H E, SILVERA I F, GOETTEL K A. Orientational phase transitions in hydrogen at megabar pressures [J]. Physical Review Letters, 1990, 64(16): 1939–1942. doi: 10.1103/PhysRevLett.64.1939
|
| [25] |
HEMLEY R J, MAO H K. Phase transition in solid molecular hydrogen at ultrahigh pressures [J]. Physical Review Letters, 1988, 61(7): 857–860. doi: 10.1103/PhysRevLett.61.857
|
| [26] |
HOWIE R T, GUILLAUME C L, SCHELER T, et al. Mixed molecular and atomic phase of dense hydrogen [J]. Physical Review Letters, 2012, 108(12): 125501. doi: 10.1103/PhysRevLett.108.125501
|
| [27] |
JI C, LI B, LIU W J, et al. Ultrahigh-pressure isostructural electronic transitions in hydrogen [J]. Nature, 2019, 573(7775): 558–562. doi: 10.1038/s41586-019-1565-9
|
| [28] |
MEIER T, LANIEL D, PENA-ALVAREZ M, et al. Nuclear spin coupling crossover in dense molecular hydrogen [J]. Nature Communications, 2020, 11(1): 6334. doi: 10.1038/s41467-020-19927-y
|
| [29] |
ASHCROFT N W. Hydrogen dominant metallic alloys: high temperature superconductors? [J]. Physical Review Letters, 2004, 92(18): 187002. doi: 10.1103/PhysRevLett.92.187002
|
| [30] |
LI Y W, HAO J, LIU H Y, et al. The metallization and superconductivity of dense hydrogen sulfide [J]. The Journal of Chemical Physics, 2014, 140(17): 174712. doi: 10.1063/1.4874158
|
| [31] |
DROZDOV A P, EREMETS M I, TROYAN I A, et al. Conventional superconductivity at 203 Kelvin at high pressures in the sulfur hydride system [J]. Nature, 2015, 525(7567): 73–76. doi: 10.1038/nature14964
|
| [32] |
DUAN D F, LIU Y X, TIAN F B, et al. Pressure-induced metallization of dense (H2S)2H2 with high- Tc superconductivity [J]. Scientific Reports, 2014, 4: 6968. doi: 10.1038/srep06968
|
| [33] |
PENG F, SUN Y, PICKARD C J, et al. Hydrogen clathrate structures in rare earth hydrides at high pressures: possible route to room-temperature superconductivity [J]. Physical Review Letters, 2017, 119(10): 107001. doi: 10.1103/PhysRevLett.119.107001
|
| [34] |
LIU H Y, NAUMOV I I, HOFFMANN R, et al. Potential high- Tc superconducting lanthanum and yttrium hydrides at high pressure [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(27): 6990–6995. doi: 10.1073/pnas.1704505114
|
| [35] |
SOMAYAZULU M, AHART M, MISHRA A K, et al. Evidence for superconductivity above 260 K in lanthanum superhydride at megabar pressures [J]. Physical Review Letters, 2019, 122(2): 027001. doi: 10.1103/PhysRevLett.122.027001
|
| [36] |
DROZDOV A P, KONG P P, MINKOV V S, et al. Superconductivity at 250 K in lanthanum hydride under high pressures [J]. Nature, 2019, 569(7757): 528–531. doi: 10.1038/s41586-019-1201-8
|
| [37] |
WANG H, TSE J S, TANAKA K, et al. Superconductive sodalite-like clathrate calcium hydride at high pressures [J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(17): 6463–6466. doi: 10.1073/pnas.1118168109
|
| [38] |
MA L, WANG K, XIE Y, et al. High-temperature superconducting phase in clathrate calcium hydride CaH6 up to 215 K at a pressure of 172 GPa [J]. Physical Review Letters, 2022, 128(16): 167001. doi: 10.1103/PhysRevLett.128.167001
|
| [39] |
SUN Y, LV J, XIE Y, et al. Route to a superconducting phase above room temperature in electron-doped hydride compounds under high pressure [J]. Physical Review Letters, 2019, 123(9): 097001. doi: 10.1103/PhysRevLett.123.097001
|
| [40] |
LI B, JI C, YANG W G, et al. Diamond anvil cell behavior up to 4 Mbar [J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(8): 1713–1717. doi: 10.1073/pnas.1721425115
|
| [41] |
卢志鹏, 祝文军, 刘绍军, 等. 非静水压条件下铁从 α到 ε结构相变的第一性原理计算[J]. 物理学报, 2009, 58(3): 2083–2089. doi: 10.7498/aps.58.2083LU Z P, ZHU W J, LIU S J, et al. Structure phase transition from α to ε in Fe under non-hydrostatic pressure: an ab initio study [J]. Acta Physica Sinica, 2009, 58(3): 2083–2089. doi: 10.7498/aps.58.2083
|
| [42] |
CHENG C. Uniaxial phase transition in Si: ab initio calculations [J]. Physical Review B, 2003, 67(13): 134109. doi: 10.1103/PhysRevB.67.134109
|
| [43] |
GAÁL-NAGY K, STRAUCH D. Transition pressures and enthalpy barriers for the cubic diamond→ β-tin transition in Si and Ge under nonhydrostatic conditions [J]. Physical Review B, 2006, 73(13): 134101. doi: 10.1103/PhysRevB.73.134101
|
| [44] |
DANG C Q, LU A L, WANG H Y, et al. Diamond semiconductor and elastic strain engineering [J]. Journal of Semiconductors, 2022, 43(2): 021801. doi: 10.1088/1674-4926/43/2/021801
|
| [45] |
DANG C Q, CHOU J P, DAI B, et al. Achieving large uniform tensile elasticity in microfabricated diamond [J]. Science, 2021, 371(6524): 76–78. doi: 10.1126/science.abc4174
|
| [46] |
LIU C, SONG X Q, LI Q, et al. Smooth flow in diamond: atomistic ductility and electronic conductivity [J]. Physical Review Letters, 2019, 123(19): 195504. doi: 10.1103/PhysRevLett.123.195504
|
| [47] |
LIU C, SONG X Q, LI Q, et al. Superconductivity in compression-shear deformed diamond [J]. Physical Review Letters, 2020, 124(14): 147001. doi: 10.1103/PhysRevLett.124.147001
|
| [48] |
LIU C, SONG X Q, LI Q, et al. Superconductivity in shear strained semiconductors [J]. Chinese Physics Letters, 2021, 38(8): 086301. doi: 10.1088/0256-307X/38/8/086301
|
| [49] |
KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set [J]. Physical Review B, 1996, 54(16): 11169. doi: 10.1103/PhysRevB.54.11169
|
| [50] |
KRESSE G, JOUBERT D. From ultrasoft pseudopotentials to the projector augmented-wave method [J]. Physical Review B, 1999, 59(3): 1758–1775. doi: 10.1103/PhysRevB.59.1758
|
| [51] |
PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple [J]. Physical Review Letters, 1996, 77(18): 3865–3868. doi: 10.1103/PhysRevLett.77.3865
|
| [52] |
MONKHORST H J, PACK J D. Special points for Brillouin-zone integrations [J]. Physical Review B, 1976, 13(12): 5188–5192. doi: 10.1103/PhysRevB.13.5188
|
| [53] |
ZHANG Y, SUN H, CHEN C F. Superhard cubic BC2N compared to diamond [J]. Physical Review Letters, 2004, 93(19): 195504. doi: 10.1103/PhysRevLett.93.195504
|
| [54] |
PAN Z C, SUN H, CHEN C F. Colossal shear-strength enhancement of low-density cubic BC2N by nanoindentation [J]. Physical Review Letters, 2007, 98(13): 135505. doi: 10.1103/PhysRevLett.98.135505
|
| [55] |
PAN Z C, SUN H, CHEN C F. Indenter-angle-sensitive fracture modes and stress response at incipient plasticity [J]. Physical Review B, 2009, 79(10): 104102. doi: 10.1103/PhysRevB.79.104102
|
| [56] |
PAN Z C, SUN H, ZHANG Y, et al. Harder than diamond: superior indentation strength of wurtzite BN and lonsdaleite [J]. Physical Review Letters, 2009, 102(5): 055503. doi: 10.1103/PhysRevLett.102.055503
|
| [57] |
BARONI S, DE GIRONCOLI S, DAL CORSO A, et al. Phonons and related crystal properties from density-functional perturbation theory [J]. Reviews of Modern Physics, 2001, 73(2): 515–562. doi: 10.1103/RevModPhys.73.515
|
| [58] |
GIANNOZZI P, BARONI S, BONINI N, et al. Quantum ESPRESSO: a modular and open-source software project for quantum simulations of materials [J]. Journal of Physics: Condensed Matter, 2009, 21(39): 395502. doi: 10.1088/0953-8984/21/39/395502
|
| [59] |
TSE J S, KLUG D D, YAO Y S, et al. Structure and spectroscopic properties of dense solid hydrogen at 160 GPa [J]. Solid State Communications, 2008, 145(1/2): 5–10. doi: 10.1016/j.ssc.2007.10.018
|
| [60] |
MONSERRAT B, NEEDS R J, GREGORYANZ E, et al. Hexagonal structure of phase Ⅲ of solid hydrogen [J]. Physical Review B, 2016, 94(13): 134101. doi: 10.1103/PhysRevB.94.134101
|
| [61] |
SINGH R, AZADI S, KÜHNE T D. Anharmonicity and finite-temperature effects on the structure, stability, and vibrational spectrum of phase III of solid molecular hydrogen [J]. Physical Review B, 2014, 90(1): 014110. doi: 10.1103/PhysRevB.90.014110
|
| [62] |
MONACELLI L, CASULA M, NAKANO K, et al. Quantum phase diagram of high-pressure hydrogen [J]. Nature Physics, 2023, 19(6): 845–850. doi: 10.1038/s41567-023-01960-5
|
| [63] |
BORINAGA M, RIEGO P, LEONARDO A, et al. Anharmonic enhancement of superconductivity in metallic molecular Cmca-4 hydrogen at high pressure: a first-principles study [J]. Journal of Physics: Condensed Matter, 2016, 28(49): 494001. doi: 10.1088/0953-8984/28/49/494001
|
| [64] |
WEN L B, WU H, SUN H, et al. Profound softening and shear-induced melting of diamond under extreme conditions: an ab- initio molecular dynamics study [J]. Carbon, 2019, 155: 361–368. doi: 10.1016/j.carbon.2019.08.079
|
| [65] |
LI Z G, CHEN Q F, GU Y J, et al. Multishock compression of dense cryogenic hydrogen-helium mixtures up to 60 GPa: validating the equation of state calculated from first principles [J]. Physical Review B, 2018, 98(6): 064101. doi: 10.1103/PhysRevB.98.064101
|
| [66] |
RANIERI U, CONWAY L J, DONNELLY M E, et al. Formation and stability of dense methane-hydrogen compounds [J]. Physical Review Letters, 2022, 128(21): 215702. doi: 10.1103/PhysRevLett.128.215702
|
| [67] |
SONG X Q, YIN K T, WANG Y C, et al. Exotic hydrogen bonding in compressed ammonia hydrides [J]. The Journal of Physical Chemistry Letters, 2019, 10(11): 2761–2766. doi: 10.1021/acs.jpclett.9b00973
|
| [68] |
HEMLEY R J, MAO H K, SHEN G Y, et al. X-ray imaging of stress and strain of diamond, iron, and tungsten at megabar pressures [J]. Science, 1997, 276(5316): 1242–1245. doi: 10.1126/science.276.5316.1242
|
| [69] |
MAO H K, BADRO J, SHU J F, et al. Strength, anisotropy, and preferred orientation of solid argon at high pressures [J]. Journal of Physics: Condensed Matter, 2006, 18(25): S963–S968. doi: 10.1088/0953-8984/18/25/S04
|
| [70] |
HEMLEY R J, MAO H K. Optical studies of hydrogen above 200 gigapascals: evidence for metallization by band overlap [J]. Science, 1989, 244(4911): 1462–1465. doi: 10.1126/science.244.4911.1462
|
| [71] |
NARAYANA C, LUO H, ORLOFF J, et al. Solid hydrogen at 342 GPa: no evidence for an alkali metal [J]. Nature, 1998, 393(6680): 46–49. doi: 10.1038/29949
|
| [72] |
LOUBEYRE P, OCCELLI F, LETOULLEC R. Optical studies of solid hydrogen to 320 GPa and evidence for black hydrogen [J]. Nature, 2002, 416(6881): 613–617. doi: 10.1038/416613a
|
| [73] |
EREMETS M I, TROYAN I A. Conductive dense hydrogen [J]. Nature Materials, 2011, 10(12): 927–931. doi: 10.1038/nmat3175
|
| [74] |
ZHA C S, LIU Z X, HEMLEY R J. Synchrotron infrared measurements of dense hydrogen to 360 GPa [J]. Physical Review Letters, 2012, 108(14): 146402. doi: 10.1103/PhysRevLett.108.146402
|






 下载:
下载: